Cell culture
Human MSCs from bone marrow were purchased from Riken BioResource Research Center (Ibaraki, Japan) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM: Sigma-Aldrich, St. Louis, MO, USA) with 10% fetal bovine serum (FBS, Sigma-Aldrich). Cells were passaged four to five times before use for transplantation. HK-2 cells, a human proximal tubular cell line, were obtained from the American Type Culture Collection (Manassas, VA). These cells were cultured as described previously47.
MSCs pretreatment with IFN-γ
MSCs were pretreated with or without recombinant human IFN-γ (PeproTech, Cranbury, NJ, USA) by the following method. When MSCs reached 70% confluence, IFN-γ was added to the medium to achieve a final concentration of 10 ng/mL. After 48 h, cells were collected and subjected to in vivo and in vitro analyses.
Experimental animal model
Male Sprague Dawley (SD) rats (8 weeks old) were purchased from Charles River Laboratories Japan (Yokohama, Japan). Experimental procedures were approved by the Institutional Animal Care and Use Committee of Hiroshima University (Hiroshima, Japan) (Permit Nos. A15-66 and A17-75) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th ed, 2010 (National Institutes of Health, Bethesda, MD, USA). This study is reported in accordance with ARRIVE guidelines. To establish the animal model, SD rats were randomly divided in 6 groups (n = 5 in each group): sham, PBS (control), MSCs, IFN-γ MSCs, NC siRNA/IFN-γ MSCs and IDO1 siRNA/IFN-γ MSCs groups. All procedures were performed under anesthesia with injection of agents composed of midazolam, medetomidine, and butorphanol. Right nephrectomy was performed 7 days prior to IRI of the left kidney. Renal IRI was induced by transiently clamping the unilateral renal artery. After a laparotomy was performed, the left kidney was exposed. Next, the renal pedicle was clamped by atraumatic vascular clamps for 45 min, followed by reperfusion on a heating blanket. After reperfusion, phosphate-buffered saline (PBS, vehicle), control MSCs, or IFN-γ MSCs (5 × 105 cells/rat) were injected through the abdominal aorta clamped above and below the left renal artery bifurcation. At 7 or 21 days post-injection, rats were sacrificed and their kidneys were collected to evaluate inflammation and fibrosis.
Immunohistochemistry analysis
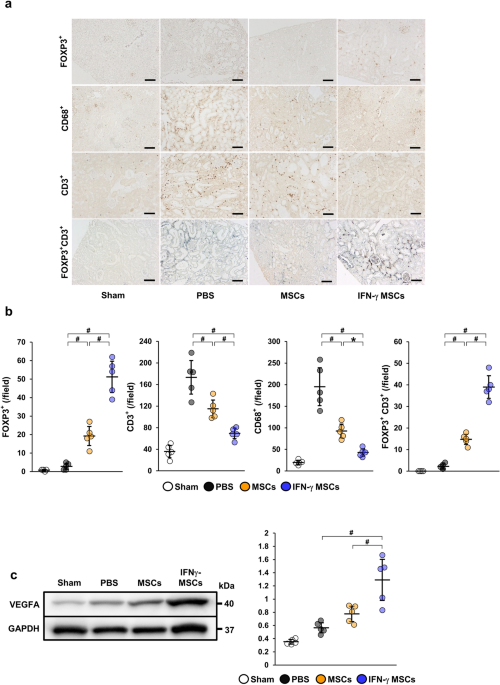
Immunohistochemical staining was performed according to previously described methods47 using the following primary antibodies: mouse monoclonal anti-Foxp3 (Abcam, Cambridge, UK), rabbit polyclonal anti-CD3 (Dako, Glostrup, Denmark), mouse monoclonal anti-rat CD68 (Serotec, Oxford, UK), and rabbit polyclonal anti-collagen type I (Abcam). FOXP3-, CD3- and CD68-positive cells, as well as areas positive for α-SMA and collagen type I staining, were assessed using ImageJ software (version 1.53 s, NIH) by examining five randomly selected fields (100× magnification) of the cortex.
Immunohistochemistry analysis (double immunostaining)
Double immunostaining was performed according to the following methods. Sections of formalin-fixed, paraffin-embedded tissues (4 μm thick) were de-paraffinized, subjected to heat-mediated antigen retrieval in citric acid buffer at 98 °C for 40 min, and then blocked in 5% skim milk at room temperature for 1 h. They were incubated with anti-FOXP3 antibody (Abcam) overnight at 4 °C, followed by incubation with the appropriate secondary antibody (DAKO) at room temperature for 1 h, and then incubated with 3,3′-diaminobenzidine (Sigma-Aldrich) at room temperature for 5 min. After that, they were heated again in EDTA buffer (pH 9.0) in the same way. They were then blocked in 2.5% normal horse serum (ImmPRESS Horse Anti-Rabbit IgG Polymer kit; Vector Laboratories, Riverside, CA, USA) at room temperature for 20 min, followed by incubation with anti-CD3 antibody (Abcam) overnight at 4 °C. They were incubated with the secondary antibody (ImmPRESS Horse Anti-Rabbit IgG Polymer kit; Vector Laboratories) at room temperature for 30 min and then incubated with working solution prepared with Vector SG Peroxidase (HRP) Substrate Kit (Vector Laboratories) at room temperature for 5 min.
Histological analysis
Sections of formalin-fixed, paraffin-embedded tissues (2 μm thick) were stained with Masson’s trichrome to assess fibrosis. Areas of interstitial fibrosis were assessed using Lumina Vision (Mitani, Osaka, Japan) by examining five randomly selected fields (100× magnification) of the cortex.
Western blot analysis
Sample collection and western blotting were performed as previously reported36,47 with the following primary antibodies: anti-VEGFA antibody (Abcam), mouse monoclonal anti-α-SMA (Sigma-Aldrich), rabbit monoclonal anti-TGF-β1 (Abcam), IDO1 polyclonal antibody (Proteintech, Rosemont, IL, USA), mouse monoclonal anti-Foxp3 (Abcam), rabbit polyclonal anti-CD4 (Abcam), and mouse monoclonal anti-GAPDH (Sigma-Aldrich). Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Dako) or goat anti-mouse immunoglobulin G (Dako) were used as secondary antibodies. SuperSignal West Dura or Pico Systems (Thermo Fisher Scientific, Waltham, MA, USA) were used to detect signals. The intensity of each band was analyzed by ImageJ software and standardized to the level of GAPDH.
Preparation of conditioned medium
To generate conditioned medium (CM) from untreated MSCs (control MSCs-CM) and IFN-γ MSCs (IFN-γ MSCs-CM), human MSCs (3 × 105 cells/dish) were seeded in 10-cm dishes and cultured in DMEM containing 10% FBS. When the cells reached at least 70% confluence, the medium was replaced with fresh medium with or without 200 ng/mL recombinant human IFN-γ (PeproTech). After 48 h, the culture medium was replaced with DMEM containing 0.1% FBS, which was collected after 48 h.
Quantitative real-time polymerase chain reaction (qRT-PCR)
RNA extraction and real-time reverse-transcription PCR were conducted according to previously described methods47. Specific primers and probes for human IDO1 (assay ID: Hs00984148_m1), and human β-actin (assay ID: Hs99999903_m1) were obtained as TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). mRNA levels were normalized to the level of β-actin.
Enzyme-linked immunosorbent assay (ELISA)
ELISA analysis of IDO (R&D Systems, Minneapolis, MN, USA) was performed according to the manufacturer’s protocol. Concentrations were normalized to the total protein content.
Isolation of human naïve CD4 T cells
Human peripheral blood mononuclear cells (PBMCs; Biosciences, Berkeley, CA, USA) were suspended with the buffer formulated as MACS® BSA Stock Solution (Miltenyi, Bergisch Gladbach, NRW, Germany) and autoMACS® Rinsing Solution (Miltenyi). Cells were labelled with a Naïve CD4 + T Cell Isolation Kit II (Miltenyi) according to the manufacturer’s protocols. Naïve CD4-positive T cells were sorted by negative selection using LS columns (Miltenyi) and MidiMACS™ (Miltenyi), and then collected.
Regulatory T cell induction
Naïve CD4 T cells (1 × 106 cells/mL) were cultured in RPMI-1640 (Solarbio, Beijing, China) plus 0.1% FBS (Thermo Fisher Scientific) with MSCs-CM or IFN-γ MSCs-CM at a RPMI-1640:CM ratio of 1:1. Next, Dynabeads human T cell activator CD3/CD28 (Thermo Fisher Scientific) was added at a bead:cell ratio of 1:1, along with animal-free human recombinant IL-2 (ProteinTech) at a concentration of 300 IU/mL, and cells were incubated in a humidified CO2 incubator. The medium, IL-2, and beads were exchanged on day 3, and then cells were collected on day 5.
Transfection of IDO1 siRNA
MSCs were transfected with 20 nM siRNA against IDO1 (s7426, Applied Biosystems) or negative control siRNA (4390843, Applied Biosystems) using Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific). After 24 h, transfected cells were washed and fresh complete medium was added. When cells reached 80% confluence, they were collected and subject to in vivo experiments.
Statistical analysis
Results are expressed as the mean ± standard deviations (S.D.). For multiple group comparisons, one-way ANOVA followed by Bonferroni’s post-hoc test was applied. Comparisons between two groups were analyzed by Student’s t-test. P < 0.05 was considered statistically significant.
Ethical approval and consent to participate
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Hiroshima University (Permit Nos. A15-66 and A17-75).
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-024-60928-4
