Our goal was to find E. coli TK variants with improved activity towards aromatic acceptor aldehydes 3-FBA, 4-FBA and 3-HBA and with pyruvate as the donor, starting from the best variant to date, 6M (H192P/A282P/I365L/G506A/D469T/H100L). The 3M variant (S385Y/D469T/R520Q) was previously found to accept aromatic aldehydes, but only with hydroxypyruvate as donor. Previous attempts to introduce S385Y into 4M/D469T/H473N or 4M/D469T/H473N/R520Q actually led to a loss in activity towards 3-FBA with pyruvate4. However, this mutation was not previously tested in 6M. Other mutations of S385, including non-natural substitutions within 3M (S385X/D469T/R520Q) were also found previously to improve the activity towards aromatic aldehydes, but again only when using HPA as donor substrate33. The R520Q mutation has had a stabilising effect in several variants, often also improving activity5,31,38, but it did not improve the activity towards 3-FBA when inserted into 4M/D469T/H473N previously4. Therefore, we were interested now to explore whether any combinations of the various S385X mutations and also R520Q, could improve the activity of 6M towards aromatic aldehydes and pyruvate.
TK variant activities with aromatic substrates
A series of TK variants were generated, expressed, purified and then evaluated for their activities towards three aromatic aldehyde acceptor substrates at 50 mM, with 50 mM pyruvate as the donor substrate. The variant names, mutations and initial levels of conversion after 24 h, based on product peak areas as a fraction of total peak area for substrate and product, are shown in Table 2.
Among all eight variants TK-1, TK-2, TK-3 and TK-5C showed the greatest conversion to product after 24 h, and compared to no conversion with TK-WT. TK-3 and TK-5C also showed the greatest conversion when using 4FBA. The level of conversion for 3HBA remained relatively low with all variants. In previous work, the 6M variant (4M/H100L/D469T) gave only a 2.5% conversion of 3-FBA after 24 h. This could be increased at 1.3 mg/mL enzyme to 46.8%, but was still lower than the 62.2% achieved with the new TK-3 variant. Given the greatest activities with 3-FBA we focused further characterisation on that substrate, selecting the four most prominent variants TK-1, TK-2, TK-3 and TK-5C. We also used the conversion of 3-FBA with each TK variant to isolate the product and confirm its identity as previously4 using a combination of LC–MS and NMR (Supplementary information, Figs. S1, S2, S3).
Kinetic and stability studies of TK variants with substrate 3-FBA and pyruvate
The kinetic parameters for TK-1, TK-2, TK-3 and TK-5C were determined at 0.1 mg/mL enzyme and 50 mM pyruvate by varying the concentration of 3-FBA from 0 to 150 mM, and monitoring reactions in triplicate for up to 24 h. The kinetic parameters are shown in Table 3, alongside the specific activity at 50 mM 3-FBA, and the conversion at 24 h (taken from Table 2). For comparison, the parameters determined previously for 6 M are also shown, although the Km for 3-FBA was not determined as that experiment varied pyruvate instead. It was not possible to obtain parameters for WT-TK as no activity was detected and so rates and rate constants are set to 0, while the Km cannot be determined.
Compared to 6M, all four variants demonstrated significant increases in specific activity, Vm and kcat. The specific activity of TK-1 in particular was 400× higher than for 6M, while the Vm was 900× greater. While the kcat values for TK-1 and TK-3 were 2–3× higher than for TK-3 and TK-5C, their Km values were correspondingly higher. The resulting kcat/Km values were fairly similar for TK-1, TK3 and TK-5C at 0.52–0.6/s mM, with TK-2 at a lower value of 0.2/s mM. Clearly the two larger Km values (for TK-1 and TK-2) exceeded the studied range of 3-FBA (up to 150 mM), resulting in the larger errors on their estimated values.
The stability of the variants was also measured using thermal scanning fluorimetry with intrinsic fluorescence as the probe. TK is already known to aggregate rapidly upon unfolding44, but comparisons can still be made between variants with consistent ramping protocols, and so an apparent thermal transition mid-point (Tm) is estimated. The Tm for wild-type TK was 57.8 °C, and consistent with a previous measurement of 58.3 °C under similar conditions (0.1 mg/mL, 25 mM Tris–HCl, pH 7.5, 0.5 mM MgCl2, 0.05 mM TPP)44.
The variants all had increased Tm values, reaching 65.1 °C, and 63.7 °C for TK-3 and TK-5C respectively. TK-1 and TK-2 gave more modest increases to 59.2 °C and 61.4 °C respectively. It was previously shown that four of the mutations used within 6M increased the Tm over wild-type TK by 3.6 °C35, whereas their introduction into 3M increased the Tm by 3 °C34. These earlier experiments suggest that a significant part of the 1.4–7.3 °C increased stability in the new variants was likely to have already been present in 6M.
Overall, TK-3 was the most stable, had the lowest Km for 3-FBA and performed well in terms of catalytic efficiency (kcat/Km) and conversion (62%). However, for biocatalysis under conditions where substrate could be added in excess over Km, then the kcat and stability are more important factors. With that perspective, the variants TK-1 and TK-2 might be preferred for their elevated activity despite the slightly lower Tm values than TK-3.
It is worth comparing the impact of R520Q and S385X mutations here with their addition to previous variants. R520Q in TK-2 increased the Tm over TK-1 by 2.2 °C with no adverse impact on activity, consistent with its stabilising effects in previous studies. S385Y and S385F each had a significant impact on activity when added to 6M in the current study, leading to respective 630× and 200× increases in kcat for the reaction with 3-FBA and pyruvate. This contrasts with the previous impact of adding S385Y into the D469T/R520Q variant (to form “3M”), which improved the catalytic efficiency towards 3-FBA and HPA by significantly decreasing the Km for 3-FBA. Furthermore, when the S385Y mutation was incorporated into 4M/D469T/H473N and 4M/D469T/H473N/R520Q, it led to a loss of activity towards 3-FBA with pyruvate4. The contextual mutations were clearly very important for the effects of S385Y. S385F gave a lower kcat, but also lower Km for 3-FBA, when compared to S385Y, which mirrored the same result in S385X/D469T/R520Q variants when tested on 3-HBA and hydroxpyruvate33.
Computational docking of 3-FBA into TK-WT, 7M and 8M
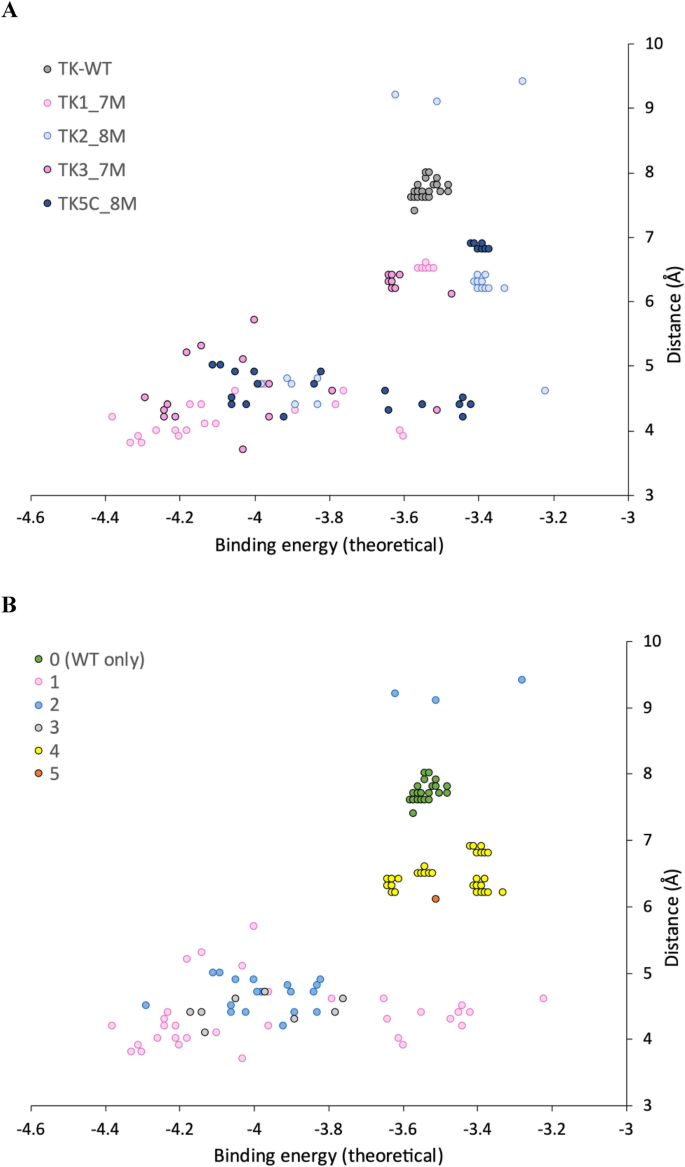
To provide some structural insights into the effects of mutations on kcat and Km, we used computational docking of the 3-FBA substrate into the active site of the TK WT, TK-1 (7M), TK-2 (8M), TK-3 (7M) and TK-5C (8M) enzymes containing the enamine intermediate expected after prior reaction of pyruvate with the ThDP cofactor. Docking of the 3-FBA was performed in Autodock 4.2 which allowed both pose clustering (based on energy and RMSD for each pose) and an analysis of the relative populations of poses obtained for each cluster, from a total of 30. To analyse each pose and cluster in more detail, we calculated the distance, d, between the TPP enamine carbanion and the C-atom of the –CHO group of 3-FBA, to approximate the likelihood that a given pose was catalytically productive (not accounting for dynamics or angle of attack).
The poses for each variant are mapped in Fig. 2, and docking parameters shown in Table 4. The calculated cluster-averaged binding energies were all in the range − 3.2 to − 4.4 kcal/mol, indicating broad energetic similarity between substrates in all clusters such that all could potentially provide sufficient binding for catalysis. However, not all binding orientations and positions would necessarily be productive for catalysis, and significant populations of non-reactive complexes in the actual enzyme could contribute to either an increase in the apparent Km or even lead to substrate inhibition. The maximum RMSD within each cluster varied from 0.1 to 1.0 Å, indicating a small combined degree of variance in substrate position, orientation and conformation within each cluster.
Distribution of binding energy and reactive distance for 3-FBA docking poses. Distributions are shown by (A) TK variant and (B) Docking cluster.
The docking produced two or three distinct clusters for each of the variants, but only a single cluster for WT (Table 4). We also analysed all poses together by visualising within aligned structures to determine structural similarities and differences between clusters from each variant. This revealed that across the four mutant variants there were actually only four clusters in total, with a few outlying poses, and that these were all distinct from the only cluster (cluster 0) in WT. Cluster 0, found only in WT, and for all WT poses, was unreactive with 7.5 < d < 8 Å. Results from Autodock Vina were consistent with Autodock 4.2 (Average d = 7.2 Å), although.
Vina also identified a rare pose with d = 4.8 Å suggesting catalysis might be theoretically possible in WT-TK but achieving the correct substrate binding may be frustrated due to binding predominantly into a non-productive location.
Of the four clusters in variants, cluster 4 was unreactive with 6.1 < d < 7.0 Å. TK2 contained the fewest potentially reactive poses (23%), with 67% in cluster 4. A further 10% for TK2 were in cluster 2b, a subcluster of cluster 2 but with a 180° relative rotation in the plane of the 3FBA ring leading to d > 9 Å. Clusters 1, 2 and 3 could be considered as “reactive”. Cluster 1 was preferred by TK1 and TK3, (even though TK3 found a single pose in cluster 2 with a lower binding energy). TK1 and TK3 each retained the wild-type arginine at residue 520. By contrast, Cluster 2 was preferred by TK2 and TK5, and these two variants contained the R520Q mutation.
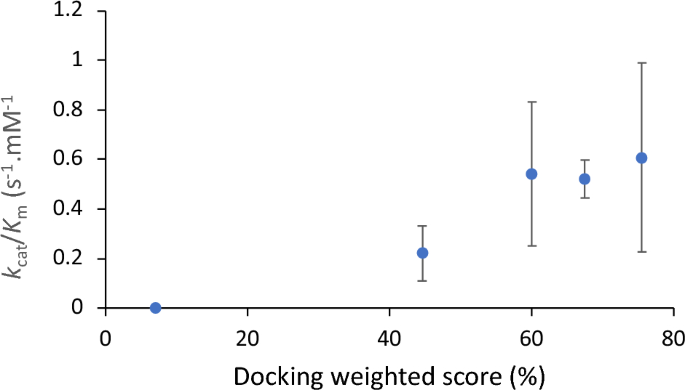
To test the relationship between population, distance and experimentally determined catalytic efficiency, we created a score (Table 4) that weighted distance by population of poses, and linearly scaled between two cut-off values such that a distance of ≤ 4 Å scored 100% and ≥ 8 Å scored 0%. Given the limitations of docking methods this analysis does not account for dynamics or specific alignment of orbitals in favourable ways, such as at the Bergi-Dunitz angle for nucleophilic attack of a carbonyl45. However, intriguingly the score scaled well with kcat/Km (Fig. 3), confirming that control of the distance to enamine, and population distributions were both major factors in the observed kinetic differences between the variants.
Correlation between kcat/Km and docking weighted score. The docking score is a linear function derived from up to three clustered populations and their mean distance between enamine and acceptor carbonyl, as Score = ∑ Popi*(2–0.25*di), limited 0–100% such that a distance of 4 Å or less scores as 100% while distances exceeding 8 Å score zero.
How do mutations influence the binding populations?
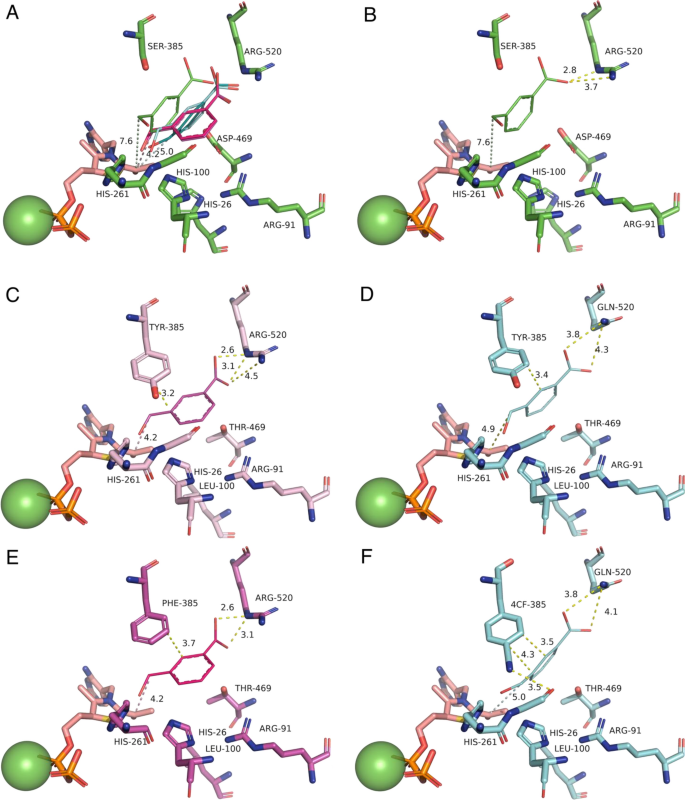
Each pose was mapped to the active site structure by aligning them via their respective TPP-enamine cofactors, to then identify common interactions with the enzyme, and the potential role of mutations (Fig. 4). In previous studies, the WT enzyme was found to be inactive on the aromatic aldehyde substrate 3FBA30,31. The earlier 3M variant which included the S385Y and D469T mutations, first introduced activity towards 3-FBA in reactions with hydroxypyruvate4,5. A crystal structure of 3M and subsequent computational substrate docking of 3FBA, 4FBA and 3HBA, revealed that D469T mutation gave a hydrophobic surface for more favourable interactions with the nonpolar acceptor substrates30. The S385Y mutation meanwhile created opportunities for π–π stacking with the new substrates, but also sterically filled the active-site space to create a more defined binding pocket. Finally, the R520Q mutation decreased the potential for interaction with the carboxylate of 3-FBA or 4-FBA. Interestingly the docking in 3M previously showed two clusters arising from the formation of two distinct binding pockets with the aromatic ring positioned on either side of D469T. 3-FBA and 4-FBA oriented into different binding pockets, whereas 3-HBA could bind into either with no obvious preference. For 3-FBA the carboxylate group was oriented towards R91 to form a salt bridge interaction.
Alignment of the top docking poses for each TK variant and WT. Alignments show (A) all five top poses aligned within the WT structure, and then top poses in their respective structures for (B) WT; (C) TK-1; (D) TK-2; (E) TK-3; (F) TK-5. 3-FBA is represented as thick lines. TPP-enamine is shown as salmon sticks. Mg2+ cofactor is shown as green spheres. Docked 3-FBA is colour coded according the top structure in each cluster as (Green) Cluster 0 in WT only; (Magenta) Cluster 1; (Blue) Cluster 2.
In the present study, docking of 3-FBA into the four key variants and WT, again revealed multiple potentially reactive binding modes, dominated by cluster 1 (Fig. 4C and E) and cluster 2 (Fig. 4D and F). Interestingly, the 3-FBA orientation observed in 3 M was no longer populated in the current variants, none of which made use of the previous interaction between 3-FBA and R91. Instead, the carboxylate groups remained oriented towards R520 in TK-1 (Fig. 4C) and TK-3 (Fig. 4E) as might be expected, but surprisingly also towards R520Q in TK-2 (Fig. 4D) and TK-5C (Fig. 4F). This orientation in TK-2 and TK-5C was supported through interactions with nearby H461 and to a lesser degree with R358, which were both also found in the WT docking of 3-FBA. However, the key difference to 3M appears to be the H100L mutation in the current variants. The imidazole sidechain of H100 interacts directly with the guanidinium of R91 in both WT-TK and 3M, and so the H100L mutation would directly modify the pKa for R91, most likely disfavouring ionisation and formation of a salt bridge to the 3-FBA substrate. This would explain the loss of the 3-FBA substrate orientation via R91 observed previously in 3M docking.
When comparing cluster 1 favoured by TK-1 and TK-3, to cluster 2 (preferred by TK2 and TK5), the key change was the R520Q mutation in TK2 and TK5. This mutation pulls 3-FBA slightly further away from the TPP-enamine (see distance d in Table 4), but also pulls it across the surface created by the D469T sidechain, thus enabling the aromatic ring face to rotate approximately 90 degrees and fit better into the cluster 2 binding pocket. By contrast, rotation of the 3-FBA ring face in TK-1 and TK-5, while maintaining the interaction with R520 would result in an unfavourable steric clash with the side chain of D469T.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-024-51831-z