Larvae of leafminer insects develop in, and feed on, parenchyma tissues between leaf surfaces of host plants, leaving behind distinctive mined tunnels and frass deposits. Leafminers can adversely affect host plant health by reducing leaf photosynthesis, increasing leaf decay, and allowing entry of diseases into hosts1. Leaf mining behaviours have evolved in four insect orders and are present in nine phytophagous fly families. They are prevalent among most of the around 3163 species of Agromyzidae Fallén, 1823 that collectively feed off over 140 families of host plants2,3,4. Various leafminer Agromyzids are agricultural pests with some being highly polyphagous across economically important host plants and are therefore significant pests of quarantine importance to international trade. Liriomyza huidobrensis (Blanchard, 1926), L. sativae Blanchard, 1938 and L. trifolii (Burgess, 1880) are prevalent among these significant pests.
These three leafminer species evolved in the Americas, but are naturalised pests in most other continents, including Australia where each has recently established in different regions5,6,7. They are collectively ranked as number 20 in the current Australian National Priority Plant Pests list8. Each pest is recognised as a significant risk to the production of a variety of economically important horticultural crops and ornamental plants. In particular, L. huidobrensis, commonly known as serpentine leafminer (referred herein as SLM), was identified during 2020 surveillance in the greater Sydney region of NSW as a novel invasive pest. SLM causes extensive foliar damage to commercial vegetable crops grown in the region including beans, cucumbers and Asian leafy greens7.
SLM can affect a broad variety of agricultural, ornamental and weed host plants in Australia7, many of which are also hosts used by the two other introduced Liriomyza pests in Australia9. The likelihood of spread of these Liriomyza pests into diverse agricultural and ecological systems and regions in Australia is high9. Subsequently affected agricultural and ornamental industries will need to develop tailored integrative pest management strategies to deal with each pest according to their biology and interactions with hosts and other leafminer species10,11,12,13. In this context, correct and rapid species identification of SLM under field conditions is critical for timely control and management of outbreaks, particularly if SLM disperses into new areas or onto novel host plants.
There are 18 naturally present Liriomyza species in Australia14. Endemic leafminer species are often not pests of agricultural concern. Cited occurrences of most of these species are scarce or only historically reported (refer Atlas of Living Australia; https://www.ala.org.au/). Direct identification of leafminer species in the field is difficult and subject to observer error. Readily observable leaf mines on host plants flags the presence of pest leafminer activity. In a few cases, the mine patterns and host identity may be indicative of a particular pest species7,15,16. In-field visual identification of adult Agromyzid leafminer species is not considered practical due to their small size (Agromyzids range in size from 0.9 to 5.6 mm) and the subtlety of morphological features used in their diagnostics. Many Agromyzid species lack a formal description, and most of the described species can only be distinguished from siblings by a few observable morphological characters. Furthermore, female adult and early instar Agromyzids generally lack species-specific features, and most species identifications are reliant on dissection of male adults and microscopic examination of their genitalia. Typically, during pest leafminer surveillance, species identifications require laboratory based taxonomic examinations of male adult flies either captured directly on hosts or raised from instars in leaf mines sampled from affected host plants. Development from egg to assayable adult in these latter instances can take 15–30 days17,18, and this can delay an alert to the presence of a priority pest and subsequent management responses.
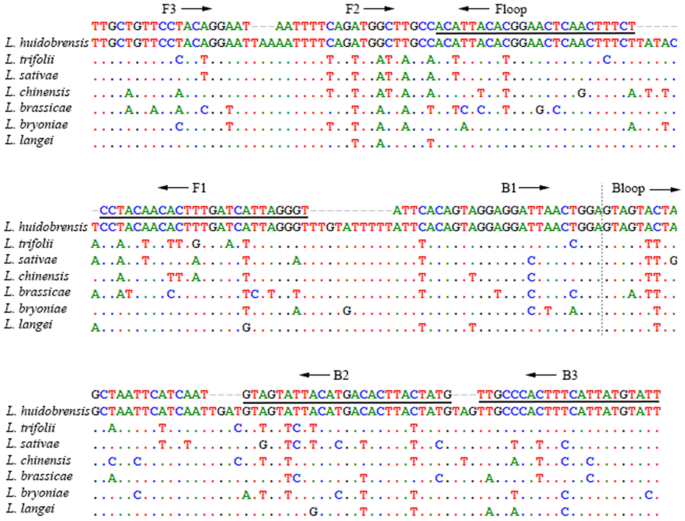
Alternatively, molecular genetic methods can provide species level identifications of leafminer flies and key Agromyzid pests. The maternally inherited mitochondrial cytochrome c oxidase subunit I (COI) gene has featured prominently as a targeted locus for molecular identification of some economically important Agromyzid species19,20,21. Nucleotide sequences of the 5’ COI DNA barcode region22 linked to vouchered specimens are reported for genetic identification of important leafminer pest species19,23. These sequence references have formed the basis for further development of laboratory and or point of need genetic diagnostic methods to identify invasive Liriomyza pests in Australia24,25 and elsewhere20,26,27. Sequences of nuclear encoded genes, including 28S and carbamoyl-phosphate synthetase 2 (CAD), reported for phylogenetic analyses of some Agromyzids3 and Liriomyza28 offer additional advantages for species identifications of pest leafminers. Comparative sequence analysis of independently inherited mitochondrial and nuclear loci were used to identify morphologically cryptic Liriomyza species28, and to test the direction of interspecific hybridisation between closely related Liriomyza species29. Recently, a quantitative Polymerase Chain Reaction (qPCR)-based molecular identification method was developed for L. huidobrensis27, addressing a critical need for Liriomyza biosecurity. However, this qPCR method has only been tested on a limited number of non-targeted species and still requires validation to confirm its applicability in Australia.
Genetic methods used for pest species identifications, such as qPCR and nucleotide sequencing, can take hours to days of laboratory processing time. This delay, coupled with delivery and registration of specimens at laboratories, increases the time required to provide an accurate identification and substantiated alert to the presence of a pest. Rapid in-field genetic diagnostics is preferable for a quick test confirmation of suspected SLM intercepts, but currently such systems are at primary stages of development or require substantive and or costly hardware.
Loop-mediated Isothermal Amplification (LAMP) is a low-cost technique for confirmation and or detection of target organisms30. LAMP incorporates a suite of oligo-primers specifically matched to the DNA of a target organism, and is designed to rapidly amplify linked copies of the target DNA. LAMP is well suited for in-field species identification of targeted pest insects, as it can test crude DNA lysates run on low-cost equipment for simple visual signalling of positive and negative test results31.
Here we report novel development and validation of sensitive LAMP assays for rapid and specific identification of SLM, against a selection of leafminer species sampled during recent surveys for SLM in Australia. Also, we report modifications of the assays to allow simplified in-field colorimetric visualisation of SLM LAMP test results.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-023-49472-9
