Gastroenteritis refers to the inflammation and infection of the gastrointestinal tract mucus membranes. The disease could cause symptoms such as mild to severe diarrhea or vomiting and even death, particularly in children1. In recent years, the use of mesenchymal stromal cells (MSCs) has been considered as a new treatment strategy in the field of disease treatment due to their potential therapeutic properties. In the current research, MSCs supernatant and nanochitosan were employed as a new treatment method for common gastroenteritis bacteria. MSCs supernatant and nanochitosan showed robust antibiofilm and antibacterial activities against gastroenteritis bacteria.
In order to synthesize hMSCsCM-ChNPs composite, it was necessary to produce ChNPs. Positively charged nanoparticles were spontaneously formed by mixing chitosan and TPP as a crosslinking agent and forming compact complexes. Intramolecular and intermolecular crosslinks were formed in ChNPs by poly-anions. In ChNPs, a magnetic attraction was created between TPP with negative charge and chitosan amino groups with positive charge19.
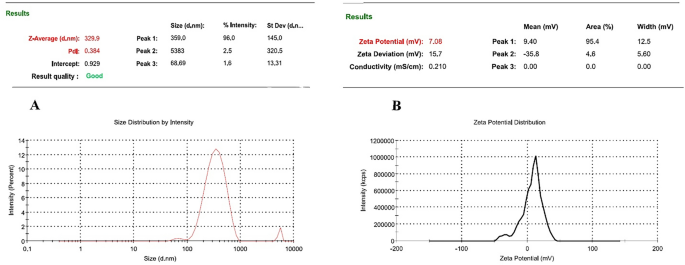
Using SEM and DLS technologies, the features of ChNPs, including morphology, zeta potential, particle size, and polydispersity index (PDI), were confirmed. No severe agglomeration was detected in nanoparticles. Morphologically, ChNPs synthesized in this study were spherical in shape as previously reported20.
Recent studies have shown that MSCs express surface molecules. The cells examined in this study were negative for CD34 and CD45 but positive for CD44 and CD73 markers, these findings are in line with MSCs features 21,22. Controlled drug delivery and release technology has become a promising strategy for controlling diseases. In this study, hMSCsCM-ChNPs composite was constructed using mesenchymal stem cells and nanochitosan as a drug delivery system. After synthesis, ChNPs were subjected to characterization and loading with MSCs supernatant. The new construct (hMSCsCM-ChNPs) was confirmed by SEM technology (Fig. 2B). The morphological features of the new (hMSCsCM-loaded ChNPs) formulation were visible via SEM. The optimal amount of protein encapsulated within hMSCsCM-ChNPs was 75%. It was found that a high amount of protein was entrapped. The protein release pattern was examined in PBS medium at various pH values, Fig. 3 shows the obtained findings. The protein release rate decreased with decreasing pH. According to the results, the release rate of mesenchymal stem cells was higher at pH 7.4 compared to pH 1.2. This high release rate at pH 7.4 may be due to the chemical properties of MSCs and ChNPs, which are mainly affected by pH, resulting in diverse release patterns. In addition, the protein release rate increased with time, the protein release rate was higher after 72 h compared to the shorter time intervals examined, indicating the long-term release of MSCs. According to these results, the limited and controlled diffusion allows the long-term release of MSCs and ChNPs. This is an important feature that could be utilized to deliver specific intestinal antigens23. The release trial showed that the synergistic effect of ChNPs and hMSCsCM improved the protein release performance. Therefore, the results of this study suggest a new idea for the treatment of gastroenteritis bacteria and the release of proteins.
Chitosan, as a cationic antibacterial agent, and its derivatives have fascinating antibacterial activities. This substance has been extensively investigated for its antimicrobial activities against fungi as well as Gram-negative and Gram-positive pathogens. The mechanisms of antibacterial activity of chitosan are as follows. Positively charged chitosan binds to the amino group of bacterial cell surface components and disturbs cell membranes, resulting in microbial cell death, or coats the bacterial surface to inhibit the leakage of intracellular compounds. Another mechanism is through the inhibition of DNA/RNA and protein synthesis5,24.
Cell therapy using mesenchymal stem cells (MSCs), as a vital part of the innate immune system, is considered as a promising treatment option for diseases. In addition to neonatal tissues, these cells could be collected from different adult tissues, including peripheral blood, inner organs, bone marrow, and adipose tissue. Some studies have shown that MSCs could exhibit immunosuppressive or immune-enhancing properties25.
Several investigations have shown that the beneficial effects of MSCs could be due to the presence of soluble proteins, secreted vesicles, and antimicrobial peptides (AMPs), also known as host-defense peptides, including hepcidin, defensin-2, human cathelicidin (hCAP-18/LL-37), and β- lipocalin 2. Recently, clinical studies have shown that BM-isolated MSCs supernatant displays antibacterial effects against some bacteria due to the presence of antimicrobial peptides. MSCs supernatant could display bactericidal activity against Gram-negative and Gram-positive bacteria, including E. coli, Pseudomonas aeruginosa, and S. aureus26.
The current study results showed that the combination of ChNPs and hMSCsCM could be used as an efficient therapeutic strategy to inhibit the growth of bacteria. This combination may have a synergistic effect. But the mechanism of action of this synergy is not well understood. Several investigations have reported the antimicrobial effects of ChNPs27,28.
It is suggested that ChNPs in this construct probably interact with structures on the surface of Gram-negative (e.g., lipopolysaccharides and proteins) and Gram-positive bacteria (e.g., peptidoglycan and teichoic acids). As a result, changes in cell membrane permeability lead to the escape of intracellular components and ultimately cell death.
In addition, the bacterial cell wall is suggested to be more labile to the infiltration of proteins secreted by MSCs (including LL37), effectors, and antimicrobial peptides. Therefore, the combination of MSCs and chitosan could play a significant role in disrupting the cell wall, inhibiting mRNA and protein synthesis, and neutralizing LPS (lipopolysaccharides). Biofilm formation in pathogenic bacteria is a protection mechanism against microbicides and antibiotics. This mechanism prevents antimicrobials from entering the bacterial cell surface. Due to the presence of polymeric matrices in bacterial biofilms, antimicrobials could not easily access the bacterial cell surface components. The antibiofilm property of chitosan is attributed to the presence of amino groups of N-acetylglucosamine monomers. Negatively charged biofilm components include: e-DNA, extracellular proteins, and EPS (extracellular polymeric substances), extracellular proteins could interact with positively charged chitosan. As a result, chitosan could pass through the biofilm and destroy the bacteria24.
In line with this study results, several current studies have shown that chitosan could be used as a potential antibiofilm agent29,30. In a study, the anti-quorum sensing (anti-QS), antibiofilm, and antibacterial activities of canine BM-derived hMSCsCM were investigated in-vitro. The results showed that canine BM- derived hMSCsCM significantly inhibited bacterial growth and biofilm formation of E. coli and S. aureus31. In another study, it was demonstrated that antibacterial peptides of human umbilical cord MSCs (hUCMSCs) influenced P. aeruginosa biofilm formation through reducing the biosynthesis of gene-encoded polysaccharide proteins32.
In the present research, MSCs supernatant exhibited a dose-dependent inhibitory activity against bacterial biofilm formation, which may be due to modulation of quorum sensing, inhibition of peptidoglycan synthesis, and disruption of microbial membrane structures. Furthermore, the integration of MSCs and chitosan could increase the interaction between these components and microbial cell membranes and inhibit the growth of Gram-positive and Gram-negative pathogens. Previous studies have shown that MSCs and hMSCsCM-ChNPs could inhibit biofilm formation of V. cholera8,33. In this study, the effects of hMSCsCM and ChNPs on biofilm formation of common gastroenteritis bacteria were evaluated for the first time.
According to these results, the combined use of ChNPs and hMSCsCM could be regarded as an adjunct treatment for bacterial gastroenteritis. In this study, a new drug delivery system was proposed for the treatment of gastrointestinal infections. hMSCsCM-ChNPs was designed and evaluated as a new treatment strategy and exhibited potent antibiofilm and antimicrobial activities against common gastroenteritis bacteria, which may be mediated by the synergistic effect of hMSCsCM and ChNPs.
Therefore, the combination of ChNPs and hMSCsCM could be used as an antibiofilm and antibacterial drug delivery tool in the treatment of intestinal infections to deal with the resistance and colonization of gastroenteritis bacteria and as a reactive antimicrobial agent both individually and in combination with existing antimicrobials to enhance their antimicrobial effects. The new formulation and its components were characterized and evaluated for the first time against common gastroenteritis bacteria. There are few studies on the direct or indirect mechanisms of action of antimicrobials against intestinal bacteria to summarize and analyze the results. Therefore, further comprehensive investigations are needed to support the efficiency of mesenchymal stem cells for clinical applications.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-024-64465-y
