A detail description of Materials and Methods is available in the online Supplement.
Ethics approval
All experiments were approved by the Animal Care and Use Committee of Nagoya University School of Medicine (Nagoya, Japan; permit No.: 30079), Fukuoka University (Fukuoka, Japan; permit No.: 1712121), Sekisui Medical Co. Ltd. (Tokyo, Japan; No.: 2018–070), or BoZo Research Center Inc. (Tokyo, Japan; No.: T180090) and were conducted in accordance with the Regulations on Animal Experiments in Nagoya University. The present study is reported in compliance with the ARRIVE guidelines (Animal Research: Reporting in Vivo Experiments).
Animals
A total of 33 male Wistar/ST rat pups were used in this study. They were obtained from Japan SLC Inc. (Shizuoka, Japan) and housed in a temperature-controlled room (23 °C) on a 12 h light/dark cycle with food and water ad libitum.
Three animals were allocated to sham used for radioisotope experiment, and 30 animals were exposed to HI. Of these, 27 were used for behavioral and histological evaluation, and 3 for radioisotope experiment.
The minimum sample size for the behavioral and histological evaluations was calculated based on the preliminary experiments to achieve an 80% power of testing with an error rate of 1.67%, assuming a 17% difference and 10% standard deviation in the cylinder test as a primary endpoint.
The total sample size was calculated as n = 24 in this case. Additionally, in our previous studies, a few rats would occasionally die, particularly during the process of creating the model, though all rats in this study survived finally. Therefore, the number of rats was set to 9 per each group for behavioral and histological evaluations.
Hypoxic–ischemic insult
Hypoxic–ischemic brain injury was induced on P7 using the modified method described by Rice et al32,54. In brief, the left common carotid artery was doubly ligated and was incised at the site between the ligatures under anesthesia with isoflurane. After 1 h rest, the pups were placed in a hypoxic environment (8% O2 and 92% N2 at 37 °C for 60 min). The sham group for radioisotope experiment underwent only anesthesia and identification of the left carotid artery without ligation or hypoxia.
Assessment of the injury with MRI
Diffusion-weighted MRI was performed 3–5 h after HI insult (P7) for the assignment of rats to the radioisotope experiment. Sham rats for radioisotope experiment did not undergo MRI. T2-weighted MRI was performed three days after HI insult (P10) for the assignment of rats to evaluation of safety, behavior, and histopathology.
The severity of brain injury was categorized into three grades using the method described by Mikrogeorgiou et al.55. (Supplemental Fig. 1a and 1b): mild (no or little hyperintensity in the parietal cortex), moderate (unilateral hyperintensity occupying the cortex and hippocampus), or severe (unilateral hyperintensity occupying the cortex and hippocampus and extending to the striatum and basal ganglia). Rats with mild HI injury were deemed unsuitable because the damage was too low, whereas those with severe HI injury were considered unsuitable because the damage was too high and could result in diffuse necrosis and ipsilateral brain collapse (Supplemental Fig. 2). Rats with moderate HI injury showed close equivalence to human HI. Therefore, moderately injured rats were adapted for subsequent experiments (Supplemental Fig. 2).
In vivo dynamics of intravenously administered nafimestrocel by using radioisotopes
51Cr labeling of nafimestrocel
Nafimestrocel, produced from human MSCs by exposing the cells to a combination of stresses, was supplied by Life Science Institute, Inc. (Tokyo, Japan)28,30. The cell concentration was adjusted to 1 × 107 cells/mL with Hank’s balanced salt solution (HBSS) for administration. Tracer (chromium-51 radionuclide, 185 MBq/mL; PerkinElmer Inc., Waltham, MA) was used for radiolabeling.
51Cr-labeled nafimestrocel administration
On P10, the pups received intravenous administration of 51Cr-labeled nafimestrocel (1 × 106 cells/body) or HBSS (0.1 mL/body) via the right external jugular vein under the anesthesia with isoflurane.
Preparation of brain samples
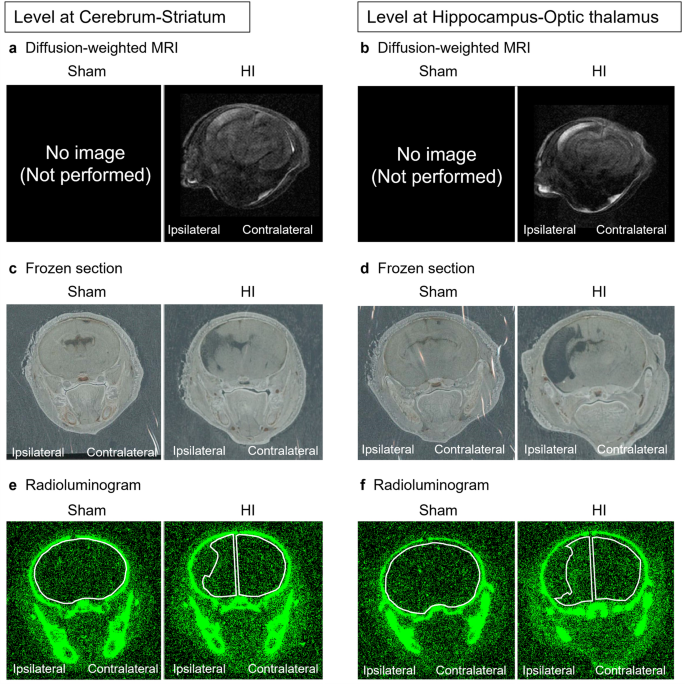
We established three time points to examine the distribution of radioactivity in rat brain: 24, 72, and 168 h after intravenous injection of 51Cr-labeled nafimestrocel. The frozen coronal brain Sects. (30 µm thickness) were prepared in two planes: one plane contained the striatum and the other contained the hippocampus and optic thalamus. The radioactivity from each section was acquired to obtain radioluminograms.
Regions of interest (ROIs) were established along the periphery of cerebral parenchyma. Radioactivity in each ROI was calculated as photostimulated luminescence (PSL) per unit area (PSL/mm2). In HI rats, the remaining parenchyma in the ipsilateral and contralateral hemispheres was evaluated. In sham rats, the whole brain was considered as the ROI to detect the smallest amount of radioactivity, and total radioactivity was calculated.
Assignment of groups
We established two time points for cell administration—three and seven days after HI insult (P10 and P14, respectively)—to verify its therapeutic effect. The cell number of injected nafimestrocel was 1 × 106 cells/body, suspended in 0.1 mL HBSS. HI rats were allocated to three groups: the M3 group (n = 9) received nafimestrocel administration on P10 and then followed by the HBSS injection (0.1 mL/body) on P14; the M7 group (n = 9) received an HBSS injection on P10 and then followed by nafimestrocel administration on P14; and the vehicle group (n = 9) received HBSS injections on P10 and P14.
Preparation of nafimestrocel for administration
Nafimestrocel for administration was prepared in the same manner as described above. Nafimestrocel was suspended in HBSS at a concentration of 1 × 107 cells/mL.
Nafimestrocel administration
The pups received an intravenous injection of nafimestrocel (1 × 106 cells/0.1 mL) or HBSS (0.1 mL/body) on P10 and P14 according to the group assignment. No immunosuppression was performed throughout this study.
Behavioral tests
Grouping for all behavioral tests and evaluations was done blindly.
Cylinder test
The cylinder test was performed to assess forelimb use preference from P36 to P38 consecutively using the modified method of Schallert et al.56. The forelimb use preference was calculated as follows: (nonimpaired − impaired)/(nonimpaired + impaired + both) × 100. The average value in each rat was used for statistical analysis.
Open-field test
The open-field test57 was conducted on P42 to assess hyperactivity. Each rat was placed in the center of an open-field chamber, and the distance traveled was recorded for 5 min using the ANY-maze Video Tracking System (Stoelting Co., Wood Dale, IL).
Water maze test
The water maze test was conducted from P53 to P57 consecutively with Morris water maze pool (Neuroscience®) and WaterMaze™ software (Actimetrics, Wilmette, IL), which was modified in accordance with Morris et al.58,59. Trial was performed three times per day. Distance traveled was recorded with software. The average from all five days was analyzed.
Pathological examination
Brains and lungs were collected at 10 weeks. Brains were weighed before tissue fixation. Paraffin-embedded brain sections including the cerebral cortex, hippocampus, thalamus, and basal ganglia were selected by referring to the Paxinos and Watson brain atlas (plate levels 92 and 93)60 and were evaluated. The sections were stained with hematoxylin–eosin (HE) and Luxol fast blue (LFB). Lung sections were randomly selected from six rats in each group and were stained with HE.
Injury evaluation
A semiquantitative neuropathological scoring system using the modified methods of previous reports61,62 (Supplemental Table) was adopted, and each hemisphere was evaluated. The mean grade in each group was used for analysis.
Microglial activation assessments
Microglial activation assessment was conducted based on the method reported by Suzuki et al. with minor modifications32. In brief, microglia (6–3 Microglia Cell Clone, COS-NMG-6-3C, Cosmo Bio Co., Ltd., Tokyo, Japan) were plated on 24-well plates at a density of 1.1 × 104 cells/cm2 (2.0 × 104 cells/well) using a medium (COS-NMGM, Cosmo Bio Co) containing 33 ng/mL recombinant mouse GM-CSF (415-ML-010/CF, R&D systems, Minneapolis, MN).
Nafimestrocel was seeded at a density of 1.8 × 104 cells/cm2 (6 × 103 cells/well) on transwell inserts (Boyden chamber: FALCON Cell Culture Insert, Corning Life Sciences, Corning, NY) and cultured in minimum essential medium Eagle, alpha modification (Thermo Fisher Scientific, Waltham, MA), with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA) and 1 ng/mL basic fibroblast growth factor (Miltenyi Biotec, Bergisch Gladbach, Germany) for 24 h. Subsequently, the inserts were transferred to the 24-well plates in which microglia were cultured for 24 h before LPS administration. The inserts with nafimestrocel were removed six days after starting the culture, and LPS (serotype O55:B5, Sigma-Aldrich, St. Louis, MO) or phosphate-buffered saline was added to microglial cultures at a concentration of 100 ng/mL. Total RNA was extracted from the microglial cultures at 3 and 24 h after adding LPS, considering microglial survival capability, and reverse transcription was performed using 200 ng of total RNA. Then, qPCR was performed by LightCycler 96 System (Roche Diagnostics, Indianapolis, IN) and KOD SYBR qPCR Mix (QKD-201, Toyobo Co., Ltd, Osaka, Japan) and following primers were used: tumor necrosis factor (TNF)-α sense, 5′-GTAGCCCACGTCGTAGCAAAC-3ʹ; antisense, 5ʹ-CTGGCACCACTAGTTGGTTGTC-3ʹ; iNOS sense, 5ʹ-CATGCTACTGGAGGTGGGTG-3ʹ; antisense, 5ʹ-CATTGATCTCCGTGACAGCC-3ʹ; β-actin sense, 5′-CGTGGGCCGCCCTAGGCACCA-3; and antisense, 5′-ACACGCAGCTCATTGTA-363.
Statistical analysis
Statistical analysis was performed by using SPSS software version 26 (SPSS Inc., Chicago, IL) and GraphPad Prism software version 9 (GraphPad Software, San Diego, CA). One-way analysis of variance, followed by Holm–Šídák’s multiple comparisons test, was used to assess body weight gain, behavioral test results, and microglial activation. Dunn’s test was used to analyze the findings of pathological examination. The value of M3 or M7 was compared with that of vehicle respectively. The Kaplan–Meier method and log-rank test with Bonferroni correction were employed to analyze the survival rate of rats. A p-value of < 0.05 was considered statistically significant. All values correspond to mean ± standard deviation.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- ChartPrime. Elevate your Trading Game with ChartPrime. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://www.nature.com/articles/s41598-023-41026-3
