The Association for the Advancement of Blood & Biotherapies or AABB recently had a very useful Q&A with the FDA on cord blood use. There are some important new things from the FDA on the marketing and use of cord blood in there.
The agency was also quite blunt in some ways about the challenges, while in others I think they overstated their effectiveness. Overall, it’s one of the more useful recent windows into the FDA’s thinking, which is not always easy to read.
Let’s dig into a couple of the key questions and answers.
Note for reference that AABB used to stand for American Association of Blood Banks and it is still made up of many blood cell product bankers and manufacturers. Quite a few members specifically produce cord blood products and supply them to various customers. Cord biologics can also include MSCs or even Wharton’s jelly from the wall of the cord.
The AABB asks FDA about cord product misuse by bank customers
The Q&A presentation, as part of the AABB annual meeting, included some excellent questions.
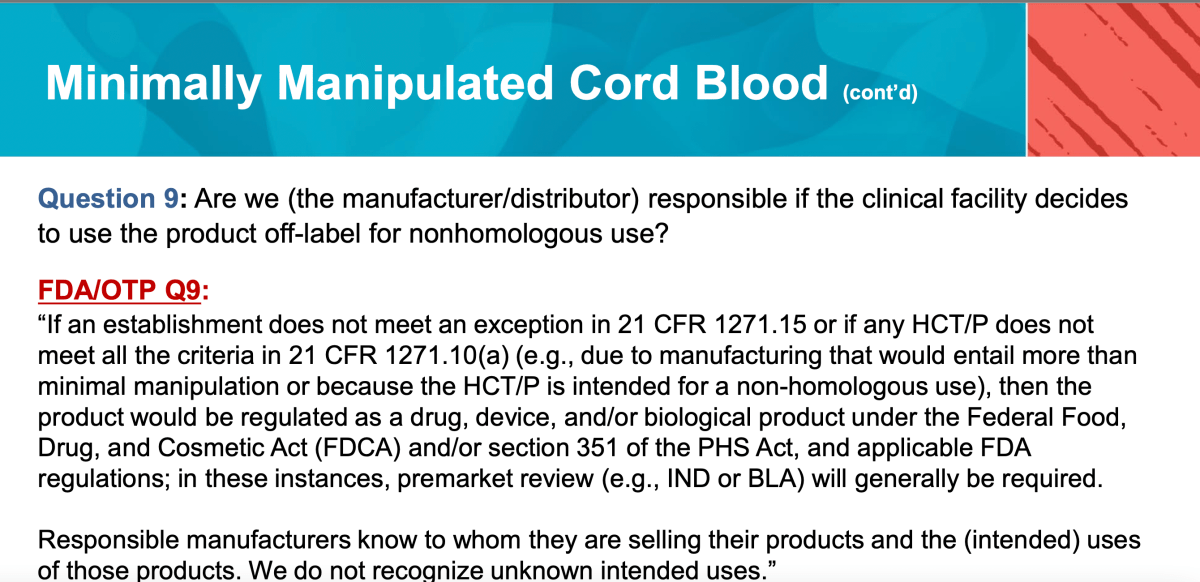
For example, AABB Q9 above:
“Are we (the manufacturer/distributor) responsible if the clinical facility decides to use the product off-label for nonhomologous use?”
In other words, can good citizen cord blood manufacturers/distributors get in trouble with the agency if their customers misuse the products?
The FDA answer here, in my reading, holds manufacturers and distributors somewhat responsible for only selling to customers who will use cord blood responsibly. That’s a big ask. For example, it seems difficult for manufacturers and distributors to anticipate all possible future uses. Customers may also drift in focus and clinical intentions over time while still maintaining stock of frozen cord materials.
Admittedly, there have been some irresponsible cord product manufacturers/distributors over the years who knowingly supply material to reckless clinics, but my impression is that these are the minority.
Also, what about the FDA’s role in oversight of this space more generally? Shouldn’t the agency be more effectively reining in most misuse after years of obvious noncompliant and even outright illegal usage? It’s just not happening. Effective oversight would have made the jobs of good citizen manufacturers and distributors far more straightforward. Right now, instead, there are hundreds of firms misusing cord products including cord blood for profit.
FDA hasn’t effectively acted on cord biologics misuse
Then there’s Question 11:
Question 11: These so called “clinics” are misleading the general public and sowing misinformation. There appear to be hundreds locally, and thousands across the country. Please update us on what FDA is doing to address these “clinics” using cord blood and other cellular material inappropriately?
Here are excerpts of the FDA’s answer and my comments/notes.
“In August 2017, FDA announced an increase in its enforcement and compliance actions against purveyors of unapproved stem cell and related products. Since that time, FDA has engaged in continuous, collaborative efforts with the U.S. Department of Justice to pursue and support FDA’s enforcement actions in federal district and appellate courts, including two injunctions and a seizure.”
Note that while the FDA has increased enforcement actions specifically in the perinatal space overall, the two injunctions and seizure it mentions (while positive actions) were not related to cord blood or other birth-related products. Rather, they were related to adipose cell products.
The FDA goes on to note the letters it has sent to non-compliant firms:
In best-case scenarios, Warning Letters and Untitled Letters prompt individuals and companies to rectify violations and commit to complying with the law going forward.
Except the best-case scenarios also seem to be the rarest outcomes.
FDA has sent 28 warning letters in cell therapy space?
They get more specific about actions in the broader cellular biologics space:
Since August 2017, FDA has issued 28 Warning Letters involving stem cell or related products that violated the Public Health Service Act (PHS Act); the Federal Food, Drug, and Cosmetic Act (FD&C Act); and/or their implementing regulations. FDA issued most of these letters following facility inspections; during these inspections, FDA investigators had documented significant violations of current good manufacturing practice requirements. In addition to the Warning Letters, FDA has issued over 40 Untitled Letters involving stem cell or related products since July 2018. Since December 2018, FDA also has issued over 600 letters to manufacturers, healthcare providers, and clinics in almost all 50 states who may be offering stem cell or related products. These letters notify recipients that they appear to be marketing a product that may be in violation of the PHS Act, the FD&C Act, and/or their implementing regulations.
There are interesting new data in this answer.
Twenty-eight relevant warning letters in this area since mid-2017 seems like a very high number compared to what I’ve seen on the FDA warning letter webpage when I search for relevant terms. Are some letters not in the public domain yet?
The 40 and 600 untitled and non-specified letters also reflect extensive correspondence between the FDA and clinics.
Looking ahead, things still seem pretty bleak on unproven clinics
Are these hundreds of letters doing much good?
The FDA finishes answering this question with mixed messages (emphasis mine):
“Finally, FDA has also been very active in promoting stakeholder education and outreach activities for patients, hospitals, healthcare practitioners, manufacturers, and clinics in this field.
While FDA’s case-specific enforcement, compliance, education, and outreach activities largely have been successful, these efforts unfortunately have not deterred the continued proliferation of those manufacturing and marketing lucrative unapproved stem cell or related products.”
It’s like they are saying in one sentence the opposite things that their efforts have largely been successful but also unsuccessful.
The unsuccessful part (no deterrence of proliferation of unproven clinics and suppliers) seems more accurate.
In the adipose cell space, FDA actions seem to depend on the outcome of their appeal of Judge Bernal’s ruling in the Cell Surgical Network lawsuit.
The paradox
However, the perinatal space is not entangled in any major litigation like that. Same with the exosome arena.
So why can’t the FDA be bolder here?
Why have there been no injunction efforts in this area?
The reality is that many clinic firms and their leaders are just not going to respond positively to letters.
In the bigger picture, there’s a frustrating paradox with the FDA in the biologics space. The better citizen you are, the more the FDA seems to expect of you, while in contrast the agency doesn’t properly oversee most bad citizens.
Time for a big change in FDA strategy
The agency needs to make a major change here as the strategy of largely sending letters isn’t working. It’s been obvious for years that their strategy isn’t work.
As I’ve said before, the FDA could starting issuing fines rather than just sending letters. Such a step would not necessarily require labor-intensive in-person inspections of firms and would send a strong message. Firms would pay attention to large fines.
Is there some reason the agency cannot issue fines here now? Historically, they’ve issued fines in many other cases.
Also, is it time to step up criminal enforcement steps against the most egregious firms and people? Many people have been hurt by clearly unlawful cell therapy marketing and use including in the cord space specifically.
The lack of effective oversight of non-compliant behavior in the biologics space it is harmful to AABB members, consumers/patients more broadly, and the larger cell therapy research field.
Related Posts
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://ipscell.com/2023/12/surprising-new-fda-remarks-to-aabb-on-cord-biologics-misuse/
