Ethics statement
The study was conducted in the Fish Physiology and Genomics department (LPGP) at INRAE (Rennes, France) fish facility. All fish were reared in the ISC INRAE LPGP fish facility, which hold full for experimental fish rearing in strict line with French and European Union Directive 2010/63/EU for animal experimentation (agreement number: D-35-238-6). The experiments and handling of fish were approved by the welfare committee of the Fish Physiology and Genomics department at INRAE (registration O-2022-01-CL) in agreement with the guidelines for care and use of laboratory animals and in compliance with French and European Union regulations on animal welfare (agreement n°005239, C. Labbé). All fish were handled for sperm collection in strict accordance with the guidelines of the Institutional Animal Care and Use Ethical Committee in Rennes LPGP (Fish Physiology and Genomics Department). CL is accreted by the French Veterinary Authority for fish experimentation (no. 005239). The animal study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org) for animal research.
Sperm collection
Medaka fish (Oryzias latipes) were from the CAB strain. The fish used in this study were 6 to 9 months old with body lengths of 3.0 ± 0.4 cm (mean ± SD). They were reared in re-circulating water system at 26 °C and the photoperiod was set at 14 h light: 10 h dark. The fish were fed twice per day with Gemma micro 500 (Skretting, Norway) and once per day with baby brine shrimps (Artemia spp.). Methods for sperm collection are described in our previous methodological study13. We used the same methods with some adjustments where needed. Briefly, for stripping, we anesthetized the male medaka in TRIS buffered tricaine methane sulfonate solution MS-222 at 225 mg/L supplemented with NaHCO3 at 450 mg/L. After anesthesia, the fish were blotted with a paper towel to dry the body. Then, the fish was placed in the holding sponge under a dissecting microscope in dorsal recumbency (belly up). The calibrated micro-capillary tube with aspirator (Drummond Scientific, Pennsylvania, USA) was attached against the cloaca of the fish. Sperm was collected by gently squeezing the abdomen with blunt end smooth forceps in a rostral to caudal motion while simultaneously sucking to collect the expelled milt into the tubes. After measuring volume and macroscopic evaluation of the milt, the sample was transferred to 1.5-mL centrifuge tube containing medium for further analysis (see the next sections for more details). Based on the breeding and housing strategy of the fish facility, the fish used for sperm collection under anesthesia were either recovered or euthanized after the procedure. For testes dissection, male fish were euthanized by immersion in a lethal dose of MS-222 at 300 mg/L supplemented with NaHCO3 at 600 mg/L. The testes were removed and separated from surrounding lipid tissues while viewing with a dissecting microscope (× 10 magnification) and transferred to 1.5-mL centrifuge tube containing sperm activation medium for immediate sperm motility analysis.
Sperm motility evaluation
Total motility was analyzed using computer assisted sperm analysis (CASA) and IVOS II system (IMV technologies) using Leja chamber slides (Leja, Netherlands) with a depth of 20 µm (Leja20, four-chamber, LOT: 481815B1) (see Supplementary Video S1). The settings for CASA software were adapted for medaka based on our previous study13. The following CASA parameters were analyzed: (1) total motility (%); (2) progressive motility (%); (3) kinematics parameters: curvilinear velocity (VCL, µm/s), straight-line velocity (VSL, µm/s), average path velocity (VAP, µm/s), linearity (LIN = VSL/VCL, %), wobble (WOB = VAP/VCL, %) and straightness (STR = VSL/VAP, %). For each sample, the sperm motility was estimated with at least three fields observed each time. Each field consisted in a 30 frames movie (camera speed 60 images/sec). Sperm were considered progressive if the straightness index (STR) was > 68% (i.e. sperm turning in circle are considered not progressive).
Sperm motility initiation study
Before testing the sperm activation with different activation media, spontaneous sperm motility was evaluated in 8 males. Sperm were collected individually by abdominal massage in a 10 µL micro-capillary tube. A drop of milt was loaded onto a clean slide glass and sperm motility was estimated at × 200 magnification using dark-field microscopy (LEITZ DMRB, Leica). In this experiment, sperm motility parameters were evaluated using different activation solutions, collecting methods and different housing conditions.
Testing activation solutions
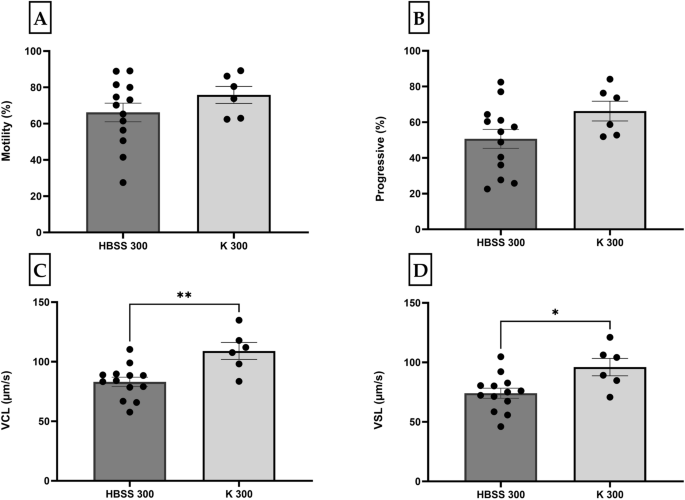
In our previous study, we found that medaka sperm were motile in HBSS (287 mOsm/kg)13, in accordance with the finding from Yang et al.2. Therefore, we chose this osmolality (~ 300 mOsm/kg hereafter as HBSS 300) for testing sperm activation in two different activation solutions (prepared in the laboratory by the authors): (1) Hanks’ balanced salt solution (HBSS 300) (137 mM NaCl, 5.4 mM KCl, 1.3 mM CaCl2, 1.0 mM MgSO4, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, and 5.55 mM glucose, pH 7.2, 300 mOsm/kg) and (2) Kurokura (K 180) (180 mM NaCl, 2.68 mM KCl, 1.36 mM CaCl2, and 2.38 mM NaHCO3). The later, Kurokura (K 180), will be referred to as K 300 in this study with regards to its osmolality. Milt was individually collected from 19 individual males and all the samples were immediately mixed with their respective activation solutions (HBSS 300, n = 13 and K 300, n = 7). Sperm was activated at room temperature (RT) by quickly mixing the sample. To obtain the optimal test temperature, the tubes of activation solutions were placed on a water bath at 28 ± 1 °C. To obtain an optimal testing concentration, the dilution factor was chosen based on the volume and density of the milt. The 1.5-mL Eppendorf tubes containing 200–500 µL activation medium were prepared before sampling. The time between activation and analysis was registered and was under 10 s for almost all the samples.
Evaluation of sperm motility in samples collected by two methods: abdominal massage (stripping) versus testes dissection
Nineteen males medaka were used to evaluated sperm motility with two different collection methods. Milt was collected by abdominal massage and analyzed as described before. Shortly after analyzing, the testes from the same 19 males were dissected and the retrieved sperm suspension was analyzed immediately after mixing the dissected testes with activation medium. The time between sample activation and analysis was recorded and was under 10 s for both collection methods. The activation medium used in this experiment was HBSS 300.
Evaluation of sperm motility in two different housing conditions: males separated from females for around one month vs. males separated from females one night prior to collection
Milt was stripped from 7 separated males and 6 males housed with females. By mixing the milt with activation medium (HBSS 300), sperm motility parameters were analyzed under 10 s after milt collection.
Sperm motility duration
To evaluate sperm motility duration, after being activated in HBSS 300, sperm suspensions from 6 individual males were stored at RT, and motility was evaluated at 0, 30, and 60 min after activation.
Sperm storage study
Short-term storage
For practical reasons, it is necessary to store sperm samples in appropriate conditions. Choice of immobilization medium, storage temperature and storage time are among factors that can affect sperm viability and quality. This is especially important when it is necessary to store the milt before in vitro fertilization (IVF), cryopreservation, microscopic and molecular assessments.
Milt from 16 individual males (four males were later excluded because of poor initial sperm quality) was collected into 16 µL of HBSS with osmolality of 600 mOsm/kg (HBSS 600), and evenly divided into 3 tubes containing HBSS 0 (n = 12), HBSS 400 (n = 12) and HBSS 600 (n = 12) to yield a final osmolality of 300, 500 and 600 mOsm/kg, respectively. The motility and velocity were evaluated at 0, 2, and 4 h post collection (hpc). In this experiment, the sperm were activated immediately after preparation of sperm suspension in HBSS 300 as initial motility (0 hpc). At the same time (0 hpc), the milt from HBSS 500 and 600, were subjected to immobilization test. At 2 and 4 hpc, the sperm in HBSS 300, 500, 600 were used to activation evaluation and sperm in HBSS 500 and 600 were further used for immobilization test. For motility evaluation by CASA, 1 µL of sperm suspension in each tube was added into a tube containing 10 µL HBSS of adjusted osmolality to yield a final osmolality of 300 mOsm/kg. For immobilization test, 1 µL of sperm suspension from HBSS 500 and 600 was added into a tube containing 10 µL HBSS with corresponding osmolalities and the percentage of immobile spermatozoa was then recorded by CASA. The samples were placed on ice during short-term storage test study.
Long-term storage (cryopreservation)
The milt was individually collected from 10 males and added to storage medium in different immobilization solutions (HBSS and Kurokura) at the osmolality of around 600 mOsm/kg. Sperm motility of fresh milt samples was evaluated by CASA after around one hour storage on ice. The sperm cryopreservation method was described previously by Depincé et al.16. Briefly, the individual sperm samples were divided into different replicates and one volume of each replicate was mixed with one volume of cryopreservation solution consisting of experimental storage solutions at 600 mOsm/kg with 16% (vol) methanol and 126mM sucrose. Aliquots (60 μL) of sperm suspension were loaded into 250-μl French straw (IMV International, Minneapolis, MN, USA) by pipetting. The samples were cooled with a cooling rate of − 10 °C/min from + 2 °C to − 80 °C in a Planer Kryo 360 controlled-rate cooler (Planer plc, UK) and straws were then transferred into liquid nitrogen. Samples were evaluated at the first day of storage in liquid nitrogen. Each straw was thawed individually in a water bath at 40 °C for 5 s, wiped dry with a paper towel, and was released into a sterile 1.5-ml centrifuge tube by cutting of the end (with the cotton plug). The samples were analyzed by CASA after thawing and cryosurvival factor (CSF) was calculated by (CSF = (%text{total motile sperm post}-text{thaw})/(%text{total motile sperm fresh})times 100). After post-thaw analysis, the rest of the sample was placed on ice, and the samples were analyzed by CASA after one hour storage on ice as described before. For post-thaw storage trials, milt samples from 10 individual males were added to immobilization solutions at different osmolalities of HBSS and Kurokura. This yielded sixteen cryopreserved samples to analyze for post-thaw storage on ice (see Supplementary Fig. S1).
Data analysis
The normality of distribution was controlled by residual and predicted values plot, normal-percentile plots, and Shapiro–Wilk test. If the p-value in the Shapiro–Wilk test was over 0.05, data were considered normally distributed. Data that were not normally distributed were transformed or analyzed by non-parametric tests, such as Mann–Whitney U. The effect of extenders, collection methods and housing conditions on motility, progressivity, VCL, and VSL was performed by independent samples t-test. One-way ANOVA analyses were conducted to find the influence of storage time on total sperm motility, progressivity, VCL, and VSL. The results are presented as mean ± S.E. The male-specific effects were handled by incorporating individual variances as random effects and nesting them within different experimental designs using a mixed model approach. All analyses were performed at a significant level of 0.05 using JMP®, Version 16 (SAS Institute Inc., Cary, NC, USA). All figures were generated using GraphPad Prism (9.0.0 for Windows, GraphPad Software, Boston, Massachusetts USA, www.graphpad.com).
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-024-65376-8
