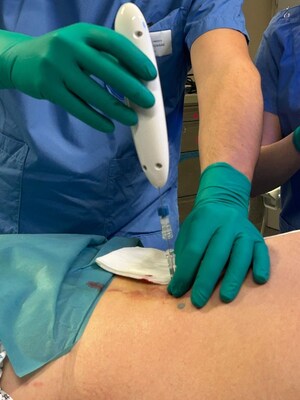
LONG BEACH, Calif., June 4, 2024 /PRNewswire/ — Single Pass and Mermaid Medical have confirmed that four patients were successfully treated with the Kronos Biopsy Closure device at MemorialCare Long Beach Medical Center.
The two liver and two kidney biopsy procedures were performed by David Tahour, MD, DABR, Chief of Interventional Radiology, who confirmed the positive results. Said Dr. Tahour “the device offers a breakthrough solution for patients undergoing percutaneous biopsy who are at high risk of bleeding complications. Knowing such a solution exists places both patients and physicians at ease and will create opportunities in the future for patients who might otherwise not been a candidate for biopsy.”
Bill Colone, CEO of Single Pass, added “the clinical success we have seen thus far in Europe, and now in the US, confirms our innovation value proposition of improving the safety of needle-guided biopsy procedures for high-risk patients.”
These cases, proctored by Dr. Tahour, also served as device training for the Clinical Specialists from Mermaid Medical, the Single Pass US national distribution partner.
The Kronos Biopsy Closure device is now commercially available through both the CE Mark under EU MDR and US 510(k) clearance.
Media Contact:
Bill Colone
6026183377
378609@email4pr.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/single-pass-and-mermaid-medical-announce-completion-of-the-first-kronos-biopsy-closure-device-procedures-in-the-us-post-fda-clearance-302163078.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/single-pass-and-mermaid-medical-announce-completion-of-the-first-kronos-biopsy-closure-device-procedures-in-the-us-post-fda-clearance-302163078.html
SOURCE Single Pass, Inc.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.biospace.com/article/releases/single-pass-and-mermaid-medical-announce-completion-of-the-first-kronos-biopsy-closure-device-procedures-in-the-us-post-fda-clearance/?s=93