Patients and sample collection
This study was approved by the Ethics Committee of Shengjing Hospital of China Medical University (2019PS012F). All the volunteers signed an informed consent after fully understanding the experimental purpose and operating procedures. All ethical regulations relevant to human research participants were followed. Tissue samples were obtained from patients aged 20–45 years without uterine myoma, adenomyoma, endometriosis, or malignant tumor. The samples of IUA were from patients who underwent transcervical resection of adhesions for grade III or above IUA (European Society for Gynecologic Endoscopy classification of uterine adhesion). The samples (about 0.1 cm3) were collected from the scar tissue at the adhesion site by using a hysteroscopic cold knife. In addition, the normal endometrial tissues were collected from women without pathological abnormality during the hysteroscopy by using an endometrial curette.
Culture and identification of hEnSCs
Primary normal hEnSCs were isolated from normal endometrium without endometriosis. The collected fresh tissues were washed 3–5 times with precooled PBS and then cut into 1-mm3 pieces. These pieces were digested with collagenase type I (Gibco, 0.1 mg/ml) for 1 h at 37 °C. Red blood cells were removed using red blood cell lysate reagent (Solarbio, China). The tissue homogenate was filtered through an aseptic 40-µm nylon strainer to remove undigested tissues and epithelial cells. The filtrate was centrifuged at 300 g for 5 min. After discarding the supernatant, hEnSCs were resuspended in DMEM containing 10% fetal bovine serum and then incubated in 5% CO2 at 37 °C. HEnSCs of passage 3 were collected for subsequent experiments.
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8) (DOJINDO, Japan) was used to assess cell viability in 96-well plates. After treatments, 10 μl of CCK-8 solution was added to each well and incubated for 2.5 h. Absorbance was determined at 450 nm using a microplate reader (BioTek, Winooski, VT, USA). The results are presented as a percent of the control results.
PCR
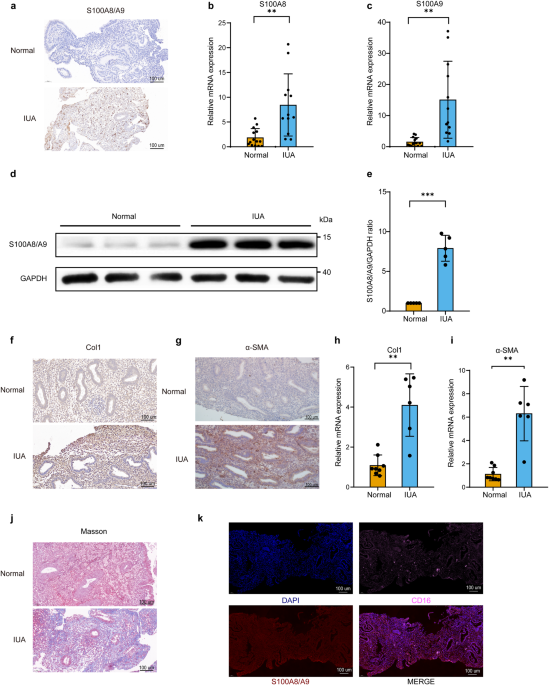
Total RNA from the endometrium was isolated using TRIzol reagent according to the manufacturer’s protocol. Amplification was performed with a fast real-time PCR system (LightCycler480II, Roche, CHE) and a SYBR Premix Ex Taq Kit (TaKaRa, Dalian, China, #RR420L). PCR primer sequences (forward and reverse, respectively) were 5’-TATCATCGACGTCTACCACAAG-3’ and 5’-TCTGCACCCTTTTTCCTGATAT-3’ for human S100A8; 5’-CCTTCCACCAATACTCTGTGAA-3’ and 5’-GGTCCTCCATGATGTGTTCTAT-3’ for human S100A9; 5’-AAAGATGGACTCAACGGTCTC-3’ and 5’-CATCGTGAGCCTTCTCTTGAG-3’ for human Col1; 5’-CTTCGTTACTACTGCTGAGCGTGAG-3’ and 5’-CCATCAGGCAACTCGTAACTCTTCTC-3’ for human α-SMA; 5’-CTCTCCTCAAATCCACTGGATG-3’ and 5’-CTATCTCAGGGAGGATCAGCA-3’ for human RAGE; 5’-GACGAAGACTGGGTGAGGAATGAAC-3’ and 5’-CCTGGATGATGTTAGCAGCGATGG-3’ for human TLR4; 5’-GCCAGTGAAATGATGGCTTATT-3’ and 5’-AGGAGCACTTCATCTGTTTAGG-3’ for human IL-1β; 5’-CACTGGTCTTTTGGAGTTTGAG-3’ and 5’-GGACTTTTGTACTCATCTGCAC-3’ for human IL-6; 5’-AGCCCTGGTATGAGCCCATCTATC-3’ and 5’-TCCCAAAGTAGACCTGCCCAGAC-3’for human TNFα; 5’-CAGGAGGCATTGCTGATGAT-3’ and 5’-GAAGGCTGGGGCTCATTT-3’ for human GAPDH; 5’-TGCTGACGGATCTGGAGAGTGC-3’ and 5’-GCGTAGATGGCGTGGTAATTCCC-3’ for swine S100A8; 5’-GACCTGGACACTAATGTGGACAAGC-3’ and 5’-TCCTCGTGAGAAGCTACCGTCAG-3’ for swine S100A9; 5’-CTCAAGATGTGCCACTCCGACTG-3’ and 5’-GTCTCGCCTGTCTCCATGTTGC-3’ for swine Col1; 5’-GATTCCACCCACGGCAAGTTCC-3’ and 5’-GATTCCACCCACGGCAAGTTCC-3’ for swine GAPDH. mRNA levels were normalized to the expression of endogenous control GAPDH in triplicate and were calculated by the 2-ΔΔCt method.
Western blot
A quantity of 10 μg of protein was loaded onto a 10% gradient polyacrylamide gel, electrophoretically transferred to a polyvinylidene difluoride membrane and probed with the following primary antibodies: S100A8/A9 (1:1000, Abcam, UK), Col1 (1:2000, Proteintech, China), α-SMA (1:6000, Proteintech, China), JAK2 (1:1000, Proteintech, China), p-JAK2 (Tyr1007) (1:1000, Absin, China), STAT3 (1:2000, Proteintech, China), p-STAT3(Tyr705) (1:1000, Absin, China), RAGE(1:1000, Proteintech, China), TLR4 (1:1000, Proteintech, China), and GAPDH (1:1000, Absin, China), which was used as an internal control. Secondary antibodies were horseradish peroxidase-conjugated to mouse anti-rabbit/mouse IgG (1:5000, Proteintech, China).
Immunohistochemistry and immunofluorescence
Immunohistochemistry was carried out by using S100A8/A9 (1:1000, Abcam, UK), Col1 antibody (1:1000, Proteintech, China), α-SMA (1:500, Proteintech, China), RAGE (1:200, Proteintech, China), p-JAK2(Tyr1007) (1:100, Absin, China), p-STAT3 (Tyr705) (1:100, Absin, China), CK18(1:1000, Proteintech, China), and vWF (1:1000, Proteintech, China). In brief, formalin-fixed, paraffin-embedded tissues were cut into 5-μm thickness and subjected to deparaffinization and rehydration. Following antigen retrieval, tissue sections were incubated with primary antibody overnight at 4 °C. After washed with PBS, sections were incubated with biotinylated secondary antibody for 1 h at room temperature. A DAB kit was employed as the chromogen and slides were counterstained with hematoxylin.
After deparaffinization, the sections were heated in EDTA for antigen retrieval and then blocked with BSA for 30 min at room temperature. The sections were then incubated with two kinds of primary antibodies at 4 °C overnight in a humidified chamber: CD16 antibody (1:5000, Servicebio, China) and S100A8/A9 antibody (1:5000, Abcam, UK). The next day, the sections were incubated with secondary antibodies for 50 min at room temperature. The secondary antibodies used were goat anti-rabbit Cy3 and goat anti-rabbit iF647 (both from Servicebio, China, diluted at 1:300). The nuclei were stained with DAPI for 10 min.
Cell immunofluorescence
The cells were fixed with 4% paraformaldehyde, permeabilized in phosphate-buffered saline (PBS) containing 0.1% Triton X-100, and blocked in 10% goat serum albumin in PBS. The cells were incubated with one of the following antibodies: Col1 antibody (1:500, Proteintech, China), α-SMA antibody (1:200, Proteintech, China), STAT3 antibody (1:500 in PBS, Proteintech, China), GFP antibody (1:100, Proteintech, China), in combination with goat anti-rabbit secondary antibody conjugated with CoraLite594 (1:500, Proteintech, China). Nuclei were counterstained with DAPI (Solarbio, China).
MenSCs isolation and culture
MenSCs isolation and culture were performed as we previously described10. Briefly, menstrual blood samples were collected on the second day of menses and transferred onto Ficoll, where they were fractionated using density-gradient centrifugation. The purified mononuclear cells obtained from the central cell layer were then washed and cultured in tissue culture bottles using Chang’s medium. The cells were incubated at 37 °C in an atmosphere containing 5% CO2, and the medium was changed every 3 days until the adherent cells reached 80–90% confluence. Finally, the cells were passaged using trypsin.
Establishment of IUA model of Bama miniature pig and local injection of MenSCs into endometrium
Four 9-month-old female Bama miniature pigs from the Beijing ShiChuang miniature pig breeding base, weighing between 18 and 20 kg, were housed in a controlled environment with a temperature range of 20 °C ~24 °C, relative humidity of 40% ~ 70%, and a light cycle of 12 h light and 12 h dark. The pigs were given free access to food and water. Electrocautery injury was utilized to establish the Bama miniature pig IUA model. Each pig had one internal control with two completely separated horns; one horn served as the electrocautery injury group (EI, n = 4), and the other served as the normal control group (Control, n = 4). Prior to surgery, the pigs were fasted and deprived of water for 12 h. Tiletamine–Zolazepam injection (25 mg/Kg) was given by intramuscular injection for induction of anesthesia, and anesthesia was maintained with isoflurane through the respirator mask. A 5–8 cm longitudinal incision was made in the middle of the lower abdomen to expose the uterus, and one cornu uteri was selected as the EI group. The entire myometrium was longitudinally cut along the opposite side of the uterine mesangium, and a 1–2 cm incision was made to expose the endometrium. Electrocautery was used to damage the endometrium, and the intensity of electrocautery was set at 30 W, 40 W, 50 W, 60 W, and 70 W, with corresponding groups named EI30, EI40, EI50, EI60, and EI70, respectively. The electrotome was run at approximately 0.5 cm/s, and each part was electrocautered three times. The uterine incision was sutured with a 4-0 absorbable suture. The normal control group consisted of the untreated part of the contralateral uterine horn. The abdominal cavity was repeatedly rinsed with normal saline, and the abdomen was closed layer by layer. The target uterine tissue was excised by laparotomy at 7 days, 21 days, and 35 days after the electrocautery injury operation. One full-layer uterus with a thickness of approximately 0.5 cm was obtained from each group and fixed in paraformaldehyde. The endometrium was excised from the remaining tissue and stored in RNAlater storage solution at −80 °C for future analysis.
In the study investigating the potential of MenSCs in preventing IUA, a total of sixteen 9-month-old female Bama miniature pigs were included and categorized into four groups. The first group underwent electrocautery injury (EI) with a power of 70 W. The second group, EI+MenSCs, received electrocautery injury with 70 W power followed by multiple injections of 1 ml containing 2 × 106 MenSCs into the endometrial layer at the site of injury. The third group was the sham group, in which the endometrium was exposed and sutured without any additional intervention. Lastly, the fourth group was the control group, which involved selecting the uterus without any treatment as the normal control group. Thirty-five days after electrocautery, endometrial images were captured using a flexible hysteroscope provided by L.H.54.
All experimental procedures were approved by the Animal Experimental Ethics Committee of Shengjing Hospital of China Medical University(2021PS529k). We have complied with all relevant ethical regulations for animal use.
Endometrial thickness measurement, endometrial gland, and capillary count
H&E-stained slices were photographed, and the endometrial thickness was then measured from four directions of each uterine cross-section using Image J software to calculate the mean thickness. CK18-stained sections were photographed, and the number of glands was counted in six randomly selected fields. vWF-stained sections were also photographed, and the number of capillaries was counted in six randomly selected fields. Complete brown endothelial clusters and brown epithelial cell clusters (positive for vWF and CK18 immunohistochemistry, respectively) were counted as independent capillaries and glands, respectively.
Masson staining and determination of collagen volume fraction (CVF) in endometrium
Masson’s trichrome staining was performed according to the kit manufacturer’s instructions (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China). Six fields were randomly selected for each Masson section. Image J software was used to calculate the percentage of the dark blue area of collagen staining positive in each field relative to the total tissue area, and the average value was taken.
Statistics and reproducibility
All data were analyzed with GraphPad Prism version 8.0 (GraphPad Prism Software, Inc., CA, USA) and are presented as means ± SD. Comparisons between two groups were performed using an unpaired two-tailed t test or unpaired t test with Welch’s correction. Comparisons between three or more groups were performed using ordinary one-way ANOVA with Tukey’s or Dunnett’s multiple comparison test, Welch’s ANOVA test with Dunnett’s T3 or Tamhane’s T2 multiple comparisons test. A P value < 0.05 was considered to be statistically significant. To ensure reproducibility, a minimum of three replicates were conducted. The figure legend details sample sizes and specifies the replicates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s42003-024-05814-5
