Generating Red2cDNA: a mosaic system based on the Confetti reporter allele using CRISPR nickase-mediated targeting
We previously reported the Red2Onco system — a series of modified Rosa26-Confetti (Brainbow2.1) alleles, which enable the mosaic, ectopic expression of oncogenes in a red fluorescent protein (RFP)-labeled, clone-specific manner22. We adapted an efficient electroporation protocol to achieve the desired gene knock-in, adjacent to the RFP, using CRISPR-Cas9 nickases with Confetti embryonic stem cells (ESCs); a process that usually takes approximately two to three weeks (Supplementary Fig. 1). The targeting approach harnesses the homology-directed repair (HDR) pathway, with homology arms of 600–700 bp in length. The Red2 targeting vector contains specific restriction cloning sites for inserting the cDNA sequence — downstream and in frame with the RFP and 2A peptide — and a PGK-Blasticidin-pA cassette with an inverted orientation for efficient antibiotic selection (Supplementary Fig. 1a). The RFP protein, tdimer2, of the confetti allele consists of two segments with repeated sequences. The left homology arm contains the sequence of one dimeric unit and can therefore be inserted to replace either dimer (with about 650 bp difference in length), where the second dimer is the desirable target24 (Supplementary Fig. 1b). For the targeting experiment, we utilized a pair of sgRNAs with a Cas9-D10A nickase (which only cleaves strands that are complementary to the sgRNA) to minimize any potential off-target effects25 (Supplementary Fig. 1c). Upon electroporation, a small fraction of cells showed GFP expression from the Cas9 nickase vectors (Supplementary Fig. 1d). After 48–72 h of recovery, cells were subjected to blasticidin treatment to select for targeted clones (Supplementary Fig. 1e), which were checked by long range PCR genotyping. Verified clones were then injected into developing blastocysts to generate chimeras (Supplementary Fig. 1f). This CRISPR-mediated method of targeting enabled mosaic genetic Red2cDNA mouse lines to be generated efficiently.
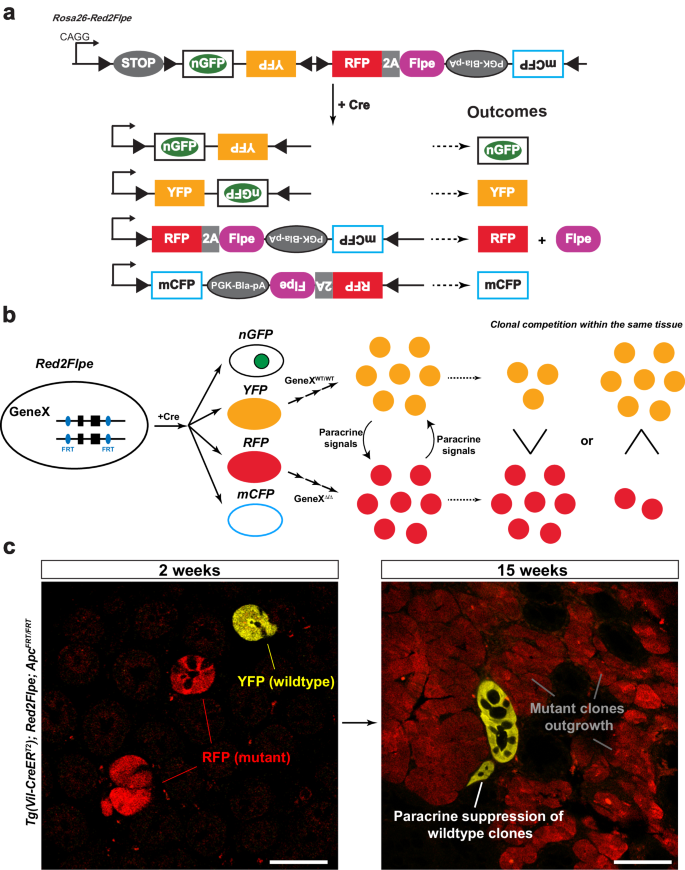
Red2-Flpe: a versatile tool that enables precise mosaic knockout with multicolor labeling
Here, we present a mosaic knockout system that involves the insertion of the Flpe recombinase sequence26 adjacent to an RFP (tdimer2) linked with a P2A peptide. This setup allows for the RFP-labeled (red), clone-specific, Flpe-mediated recombination of the target FRT allele, while ensuring that all the other fluorescent proteins remain genetically unaltered (Fig. 1a, b). We named this system, Red2Flpe. The mosaic knockout system, induced upon Cre-mediated recombination, results in the presence of both wild-type and mutant knockout cells in the same tissue. The YFP-labeled wild-type cells and RFP-labeled mutant cells undergo clonal competition that involves the secretion of paracrine factors (Fig. 1b). Depending on how the target gene knockout impacts the clonal fitness, the mutant cells could either outcompete or become eliminated, correspondingly (Fig. 1b, c). Cells carrying cancer driver mutations are known to secret paracrine factors to suppress the growth of neighboring cells22,27,28, Red2Flpe is therefore superior in accurately tracking the evolution of mutant cells and the wild-type cells in the same tissue with spatiotemporal resolution (Fig. 1c).
a Upon Cre induction, each cell (unless it is polyploid) is adapted to express one fluorescent protein color. The cells labeled with red fluorescent protein (RFP) express the Flpe recombinase, while all the other fluorescent protein colors (green/yellow/cyan) correspond to wild-type cells. b Schematic showing the interactions between YFP+ wild-type cells (GeneXWT/WT) and RFP+ mutant cells (GeneX∆/∆) through paracrine signaling. As a result, the clonal competition between wild-type and mutant cells can be quantified. c Wholemount intestines of Tg(Vil-CreERT2); Red2Flpe; ApcFRT/FRT mice from 2 and 15 weeks after tamoxifen administration. The mutant RFP cells, through the secretion of paracrine factors, outcompete the surrounding wild-type cells. The experiment in c was performed in 3 separate Tg(Vil-CreERT2); Red2Flpe; ApcFRT/FRT mice for each time point and the representative images were taken. Scale bar, 50 μm.
After activating Red2Flpe with the ubiquitous Rosa-CreERT2 line, we harvested the colon, pancreas, seminal vesicles, spleen, stomach and tongue (Supplementary Fig. 2a–f), and confirmed that both YFP and RFP were expressed in all of the analyzed tissues. Quantification of YFP+ and RFP+ clones showed no recombination bias for one fluorescent marker versus the other, as similar numbers of both clones were scored (Supplementary Fig. 2g, h). This demonstrated the utility of Red2Flpe in multiple organs and tissues with recombination rates that were suitable for mosaic lineage tracing studies.
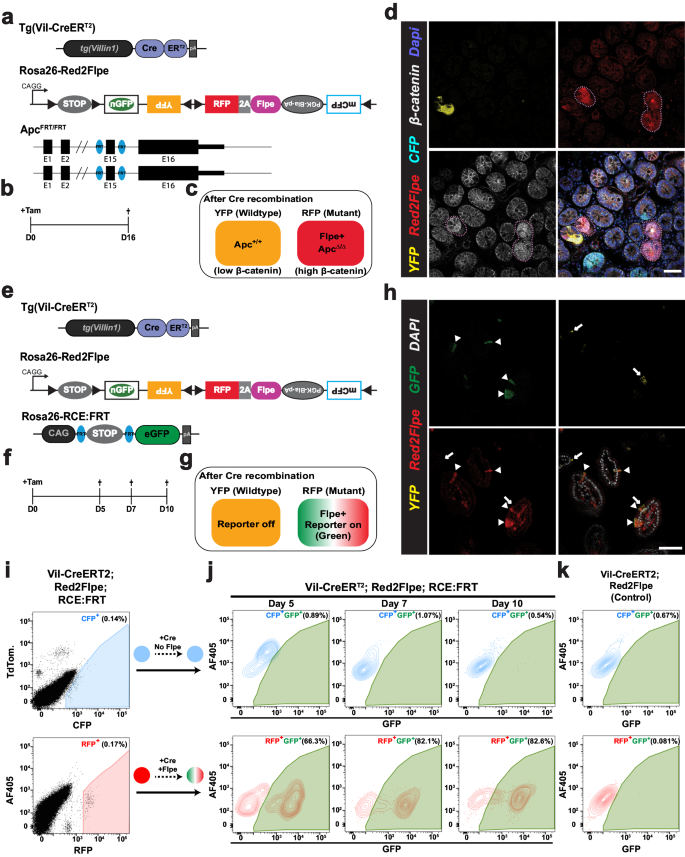
We next tested whether the expression of Flpe on the modified Confetti allele could recombine with the FRT sites on different alleles in the mouse genome, in a red clone-specific manner. In combination with the intestinal epithelium-specific CreERT2 line, Villin-CreERT2, we crossed Red2Flpe with two separate FRT lines. Firstly, Vil-CreERT2;Red2Flpe was crossed with a newly generated Apc-FRT (exon 15 flanked by FRT sites) (Fig. 2a); knockout of Apc leads to stabilized β-catenin accumulation and subsequent Wnt signaling activation29, similar to the inactivation of other Wnt negative regulators30,31. One week after the Cre-mediated labeling (Fig. 2b), the Vil-CreERT2;Red2Flp;ApcFRT/FRT mouse intestine showed that the RFP clones, specifically, displayed elevated cytoplasmic β-catenin staining (Fig. 2c, d). Secondly, the Vil-CreERT2;Red2Flpe line was crossed with an FRT-based GFP reporter line called RCE:FRT (an FRT-stop-FRT GFP reporter on the Rosa26 locus)32 (Fig. 2e). We expected overlapping GFP and RFP signals but no overlap with any other colors, if Flpe worked efficiently and specifically. After one week of Cre induction, we only observed GFP expression in the Flpe-expressing RFP+ cells (Fig. 2f–h). The rate of accumulation of RFP/GFP double positive clones was comparable to other established Confetti-based reporters8, showing a minimal delay between RFP expression and Flpe-dependent GFP expression. To assess the efficiency and specificity of Flpe/FRT recombination of Red2Flpe, primary intestinal cells were harvested and analyzed by flow cytometry at five, seven and ten days after tamoxifen injection (Fig. 2i–k). A stringent gate was set for the RFP-positive cells, as the maturation time is longer for tdimer2 (~120 min)24 (Fig. 2i). We observed an increase in the proportion of GFP/RFP double-positive cells from day five (66.3%) to day seven (82.1%) and day ten (82.6%) (Fig. 2j). There is a time lag between the point of tamoxifen injection, the observed Cre activity and the subsequent stable expression of the fluorescent reporters and Flpe recombinase; this data, therefore, suggests that Red2Flpe becomes active approximately five to seven days after the tamoxifen injection (Fig. 2j). Overall, these results indicate that Red2Flpe is a functional and efficient genetic tool that can be used to generate mosaic multicolor mouse models in vivo.
a–d Small intestine samples harvested one week after treatment with tamoxifen (2 mg tamoxifen per 20 g body weight) from a Tg(Vil-CreERT2); Red2Flpe; ApcFRT/FRT mouse. The wild-type crypts labeled with either cyan (CFP), yellow (YFP), or no color show clear β-catenin staining in the membrane with less staining in the cytoplasm. The RFP-labeled (red) crypts display an increase in the cytoplasmic fraction of β-catenin staining — indicating successful knockout of Apc and malfunction of the β-catenin destruction complex. The RFP-labeled (red) crypts and the corresponding β-catenin staining are marked with dotted lines. The experiment in a–d was performed in 3 separate Tg(Vil-CreERT2); Red2Flpe; ApcFRT/FRT mice the representative images were taken after antibody staining. Scale bar, 50 μm. e–h, Small intestine samples harvested one week after treatment with tamoxifen (2 mg per 20 g body weight) from a Tg(Vil-CreERT2); Red2Flpe; Gt(ROSA)26Sortm1.2(CAG-EGFP)Fsh mouse. The expression of RFP (red) coincides with GFP (green) but does not coincide with Confetti YFP (yellow). RFP-GFP double positive cells are marked by the triangles and the YFP cells are marked by the arrows. The experiment in h was performed in 2 separate Tg(Vil-CreERT2); Red2Flpe; Gt(ROSA)26Sortm1.2(CAG-EGFP)Fsh mice the representative images were taken. i Gating strategy of YFP, RFP and CFP cells in tamoxifen-induced Tg(Vil-CreERT2); Red2Flpe intestine. j GFP+ cells within the gated CFP+ population (top row) or the gated RFP+ population (bottom row) in an FRT-based reporter (Tg(Vil-CreERT2); Red2Flpe; Gt(ROSA)26Sortm1.2(CAG-EGFP)Fsh) mouse harvested 5, 7 or 10 days post tamoxifen administration. k GFP+ cells in the control mouse (Tg(Vil-CreERT2); Red2Flpe) after tamoxifen administration. For each time point in i and j, the intestines from 2 mice of Tg(Vil-CreERT2); Red2Flpe; Gt(ROSA)26Sortm1.2(CAG-EGFP)Fsh and one control mouse of Tg(Vil-CreERT2); Red2Flpe were profiled and showed similar results.
Short Conditional intrON (SCON) facilitates the generation of FRT-based conditional knockout mouse lines with a one-step zygote injection
As Cre-loxP was found to work more efficiently than the wild-type Flp, most of the murine conditional knockout (cKO) lines were made using the Cre-loxP system. Conditional mouse lines are often generated by in vitro ESC targeting, followed by a blastocyst injection to acquire chimeric mice, and final germline transmission; this process takes at least six months. Despite efforts to improve the efficiency of the zygote injections, using CRISPR-based knock-in with a long single-stranded oligonucleotide (ssODN)33, the process remains technically challenging.
To facilitate cKO mouse generation, we recently developed an artificial intron-based approach that uses a Short Conditional intrON (SCON), which is just 189 bp in length23. This method only requires a synthesized oligo template, a synthesized gRNA, and a commercially available Cas protein and/or mRNA. All these components are injected into zygotes to generate a cKO. SCON has a neutral effect following the initial insertion of the target gene, and induces the expected loss of function effect upon recombination in vivo. SCON, therefore, offers an alternative but efficient way to generate cKO alleles using a method that is as simple as CRISPR-based ‘tagging’.
We reasoned that the loxP recombination sites used with SCON could be exchanged with FRTs, for compatibility with the Red2Flpe mosaic knockout system. The SCON-FRT system works in the same way as the SCON-loxP system — which consists of a splice donor, branch point, and splice acceptor — with SCON acting as a functional intron. With this system, two FRT recombination sites flank the branch point; the removal of the branch point upon Flp-mediated recombination then abrogates the SCON’s intronic function, causing it to be retained in the mature transcript after splicing. The remaining 55 bp SCON intron sequence contains potential stop codons that can cause premature termination of translation and subsequent truncation of the target protein (Fig. 3a).
a Schematic diagram of SCON-FRT in an eGFP overexpression construct, including eGFP, eG-SCON-FRT-FP and recombined eG-SC-FRT-FP. SD (splice donor), BP (branch point), SA (splice acceptor). b Brightfield and fluorescent images of HEK293T cells 24 h after transfection. c GFP fluorescence level of HEK293T cells 48 h after transfection. Red: intact eGFP; Blue: eG-SCON-FRT-FP; Orange: recombined eG-SC-FRT-FP. d GFP fluorescence level of HEK293T cells with integrated eG-SCON-FRT-FP before (blue) and after (orange) transfection of a Flp-expressing plasmid. Experiments in c and d were performed twice and in two separate cell clones, which showed similar results. Violin and box plots indicate the distribution of data where minimum and maximum values are presented. The boxplot represents the central 50% of data points and the thickened line marks the median value. Source Data relevant to this Figure are provided with this paper in Source Data file.
We next tested whether the SCON-FRT system had a similar neutral effect on gene expression, as with SCON-loxP. We transfected HEK293T cells with either intact eGFP; eG-SCON-FRT-FP (eGFP with a SCON-FRT insertion); or the recombined form, eG-SC-FRT-FP (Fig. 3b). We found that the intact eGFP and the two different eG-SCON-FRT-FPs, with either wild-type or F3 FRT sites, had comparable GFP levels; however, the wild-type FRT version slightly out-performed the F3 FRT version (Fig. 3c). With the recombined forms, the level of GFP fluorescence was not detectable, indicating loss of expression (Fig. 3b, c). We also tested whether SCON-FRT could be efficiently recombined in mammalian cells upon Flp expression. We generated mouse ESCs with constitutive expression of eG-SCON-FRT-FP, using the piggyBac transposon system. After transfecting an Flp-expressing plasmid, we found that the level of GFP diminished significantly, which confirmed the compatibility of the SCON-FRT for Flp/FRT-based recombination system in mammalian cells (Fig. 3d). Therefore, we concluded that the SCON-FRT system was also neutral, like the SCON-loxP system, and it was applicable in mammalian cells.
Red2Flpe can be efficiently utilized in combination with the SCON-FRT system
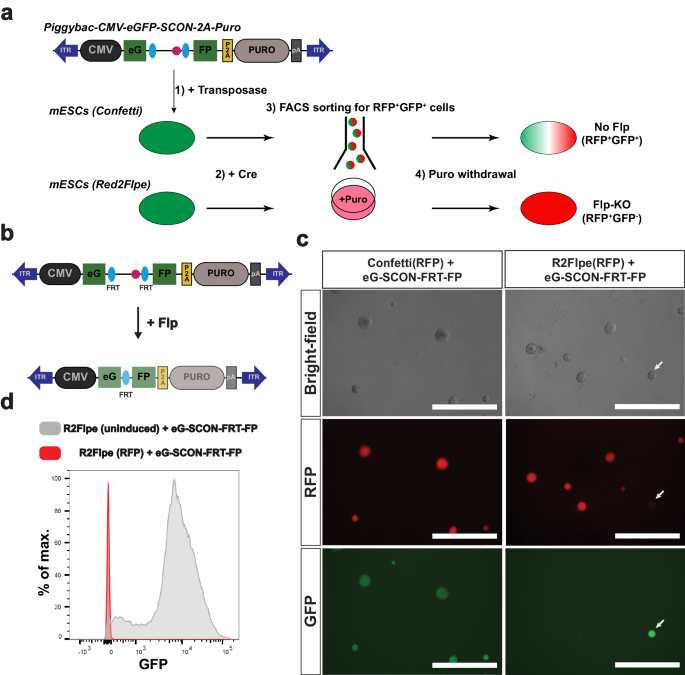
Next, we assessed the compatibility of the SCON-FRT system with Red2Flpe. We made use of Confetti and Red2Flpe ESCs in combination with piggyBac-eG-SCON-FRT-FPs (Fig. 4a, b). Cells with an integrated eG-SCON-FRT-FP were selected with puromycin with the expectation that both eGFP and puromycin-resistance expression would be compromised following Flp-mediated FRT recombination (Fig. 4a, b). After selecting GFP-expressing cells, we induced the recombination of the Confetti and Red2Flpe alleles, respectively, by transfecting a Cre-expressing plasmid. Using fluorescent activated cell sorting (FACS), RFP+ cells were sorted and cultured separately from the uninduced cells. All the RFP+ colonies of Red2Flpe ESCs showed no eGFP expression compared to the Confetti ESCs, which were used as controls (Fig. 4c). Flow cytometry revealed that most of the cells in the uninduced cultures retained high levels of GFP expression, despite some transgene silencing, while all of the recombined RFP+ cells lost GFP expression completely (Fig. 4d). These results indicated that Red2Flpe could be coupled with SCON-FRT to successfully achieve conditional mosaic gene knockouts.
a Schematic of the experimental set up that involves in integration of a piggybac-eG-SCON-FP vector into the Confetti or Red2Flpe embryonic stem cells. Upon Cre recombination, the RFP + GFP+ cells are sorted and cultured in puromycin-containing media. To assess the recombination, and the loss of GFP signals, puromycin was omitted from the media. b Schematic of the piggybac vector carrying the overexpression cassette of eG-SCON-FRT-FP coupled with puromycin resistance. Both eGFP and puromycin expression are reduced following Flp/FRT recombination. c Brightfield and fluorescent images of RFP+ Confetti and Red2Flpe ESCs with integrated eG-SCON-FRT-FP. The uninduced clone that retains eGFP expression is marked by an arrow. d GFP fluorescence level of Red2Flpe ESCs with integrated eG-SCON-FRT-FP after puromycin withdrawal. Gray: uninduced Red2Flpe; eG-SCON-FRT-FP cells; Red: RFP+ cells. Experiment shown in c and d was performed twice in two separate cell clones, which showed similar results.
Sox2-SCON-FRT mouse generation via one-step zygote injection
With the knowledge that the use of SCONs would not affect basal gene expression, we generated thirteen cKO mouse lines23. We injected CRISPR-Cas9 ribonucleoprotein (RNP), Cas9 mRNA, and a 300 bp long ssODN of SCON (using either loxP sites or FRT sites, both of which were 189 bp long). We used left and right homology arms that were 55 and 56 bp long, respectively23. With the SCON approach, a complete experimental mouse line for mosaic knockout studies could be generated quickly and easily.
We propose a pipeline for generating zygotes using SCON targeting from the desired CreER and Red2Flpe lines, both in homozygosity (Supplementary Fig. 3a). From the offspring, pups that are heterozygous for the SCON-FRT knock-in can be used for mating to create further experimental lines (Supplementary Fig. 3b). The chance of acquiring the desired experimental cohort is 1/4 (Supplementary Fig. 3c) so, theoretically, it is possible to generate SCON-based Red2Flpe mosaic knockout mice by zygote injection with just two mouse generations.
One of the first SCON-FRT lines we generated was the Sox2-SCON-FRT (Sox2scon) line (Supplementary Fig. 4a). From twenty founder pups, we obtained three heterozygous SCON-FRT knock-in mice with precise integration (15% efficiency). Sox2+/scon mice can be bred to be homozygous without any noticeable developmental defects (refer to Fig. 4g, h in Wu et al.)23, which validates the utility of SCON-loxP and FRT for in vivo experiments. The mouse ESCs derived from homozygous Sox2scon/scon blastocysts expanded stably in culture. Then, following transient Flpe expression, the colonies that contained either mosaic or complete Sox2-KO cells exhibited distorted morphologies. These results were consistent with the importance of Sox2 in maintaining pluripotency (Supplementary Fig. 4b)34.
Mosaic knockout of Sox2 in adult tissues reveals its variable essentiality
Sox2 is a transcription factor that plays crucial roles during embryonic development — from the blastocyst stages to the fate-specifying stages — of many tissues35. Knockout studies revealed that Sox2 is required for proper development of the esophagus36,37. In adults, Sox2 is expressed in the esophagus and stomach, and is thought to be important for stem cell maintenance38,39. Consistently, tissue-wide knockout of Sox2 or depletion of Sox2-expressing cells leads to compromised tissue maintenance and physiology38,39. However, as widespread knockout of Sox2 in these tissues compromises their overall integrity, the exact function of Sox2 remains unclear. A mosaic analysis of Sox2 in the adult esophagus and other tissues is therefore necessary.
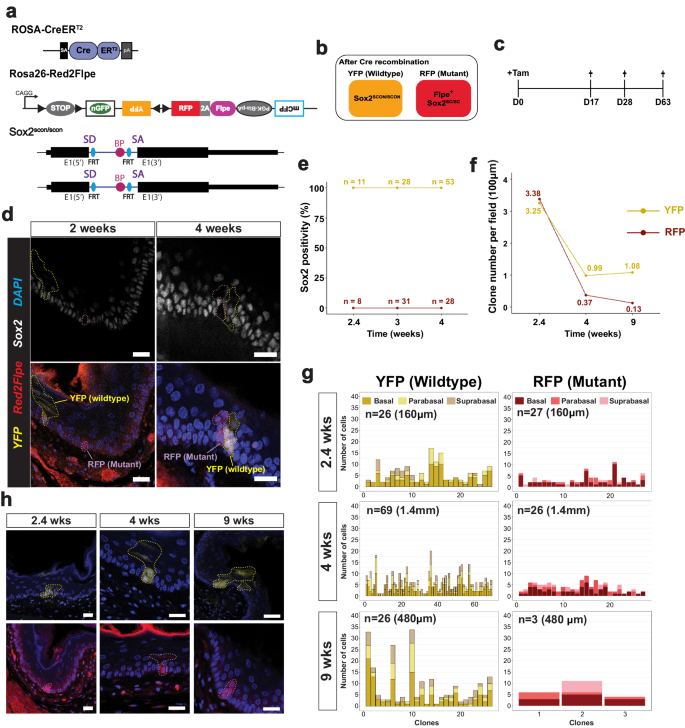
We used Red2Flpe and the Sox2scon alleles with the ubiquitous Rosa26-CreERT2 inducer line, and generated Rosa26-CreERT2; Red2Flpe; Sox2scon/scon (Red2Sox2KO) mice to investigate the function of Sox2 in the adult mouse esophagus (Fig. 5a). By administering tamoxifen to the Red2Sox2KO mice, we activated fluorescent labeling and the red clone-specific knockout of Sox2 (Fig. 5b). We then lineage traced the wild-type (yellow) and knockout (red) cells and quantified the sizes of their clones over time. If Sox2 was essential for stem cell maintenance, we would have expected that the mutant clones would be rapidly lost from the basal layer within a short period of time. By contrast, if Sox2 was not essential for stem cell maintenance, we would have expected that the mutant clones would remain present in the basal layer — and might only be lost due to their relative clonal fitness in that tissue.
a Experimental set up for mosaic Sox2 knockout in an adult mouse esophagus using the Rosa-CreERT2; Red2Flpe; Sox2scon/scon strain. b Schematic of the genotype of YFP and RFP cells after Cre administration. c A single dose of 3 mg tamoxifen was injected and the esophagus samples were then harvested on days 17, 28 and 63 after the injection. d Sox2 staining in esophageal sections at 2- and 4-weeks post-induction. The YFP and RFP clones are marked by dashed lines. Scale bar, 20 μm. e, f Quantification of Sox2+ cells and number of YFP+ and RFP+ cells per field of 100 μm at 2.4-, 4- and 9-weeks post-induction. g Quantification of spatial distribution of YFP+ and RFP+ clones at 2.4-, 4- and 9-weeks post-induction. h Representative images of YFP+ and RFP+ clones at 2.4-, 4- and 9-weeks post-induction. The YFP+ and RFP+ clones are marked by dashed lines. Experiments in c–h were performed in two mice for each time point, in which multiple fields of sectioned esophagus were imaged and quantified. Scale bar, 20 μm. Source Data relevant to this Figure are provided with this paper in Source Data file.
We collected the esophagus samples at different time points following the tamoxifen injection (Fig. 5c). We first confirmed that Sox2 expression was absent from the RFP+ clones in the esophagus at two weeks and four weeks, and checked that the YFP+ clones expressed Sox2, as expected (Fig. 5d). Interestingly, the Sox2 knockout mutant clones remained in the tissue, even after long-term tracing, which suggested that Sox2 might not be essential for stem cell maintenance in the esophagus (Fig. 5e, f). At 2.4 weeks after induction, the RFP+ and YFP+ clones were found at similar levels (Fig. 5f). However, at 4 and 9 weeks, the number of RFP+ clones was reduced compared with the wild-type YFP+ clones (Fig. 5f). We also quantified the clone sizes, and the location of the labeled cells within each clone — noting whether they were in the basal, parabasal (stratifying) or suprabasal layers. We found that the size of the RFP+ clones was consistently smaller than the size of the YFP+ clones (Fig. 5g). In addition, the RFP+ clones mainly consisted of cells in the basal and parabasal layers, whereas the cells in the YFP+ clones were more evenly distributed across all three layers (Fig. 5g, h). This suggests that, while the wild-type cells were able to efficiently self-renew and differentiate, the Sox2 mutant cells had reduced capacity for proliferation and differentiation. This eventually led to the loss of RFP+ clones from constant niche competition due to a reduction in their fitness compared to the wild-type cells.
We also utilized the esophageal organoid system to examine the behavior of Sox2 mutant clones over time. We found that the organoid-forming efficiency was slightly lower but maintained (especially in WENR+Nic condition) in Sox2 mutant cells compared to wild-type cells, with clearly smaller organoid sizes observed for all three culture medium conditions tested (WENR+Nic, ENR, or EN) (Supplementary Fig. 5). Nonetheless, Sox2 mutant organoids could still be passaged with both WENR+Nic and ENR media, suggesting persistent stem cell activity. Consistent with the in vivo lineage tracing results above, the esophageal cells that were depleted for Sox2 still retained their stem cell characteristics but exhibited reduced capacity for proliferation and differentiation. We also performed single cell RNA sequencing (scRNA-seq) of wild-type (YFP+) and the Sox2-mutant (RFP+) cells (Supplementary Fig. 6). To overcome the low induction rate and unequal cell representation, we utilized a 384 plate-based sorting method followed by scRNA-seq called SORT-seq40 to obtain comparable number of wild-type and mutant cells. We obtained 284 YFP+ cells and 303 RFP+ cells from 3 different mice 4 weeks after tamoxifen injection (Supplementary Fig. 6a). Cells were clustered based on expression of gene markers and the three major cell types41 (proliferating basal, quiescent basal and suprabasal) could be identified (Supplementary Fig. 6b, c). Wild-type (YFP+) and Sox2-mutant (RFP+) cells could be found in all three cell types (Supplementary Fig. 6b), which is in-line with our imaging results (Fig. 5). Intriguingly, YFP+ and RFP+ cells did not show significant differences across all three cell types based on marker gene expression levels41, with the exception of Krt14 and Krt5 in the quiescent basal and suprabasal layers, respectively (Supplementary Fig. 6d). Sox2 is expressed in the developing foregut and remains expressed in the epithelium of the stomach as well as the esophagus36. Therefore, we also checked whether knockout of Sox2 would have an impact on clonal fitness in the stomach epithelium. We found that both the wild-type (YFP+) and the Sox2-mutant (RFP+) glands showed comparable labeling four weeks after being induced (Supplementary Fig. 7a). We also observed the comparable presence of both wild-type and Sox2-mutant clones in the base (Supplementary Fig. 7b, c) and isthmus (Supplementary Fig. 7d, e) parts of the glands11. Overall, this indicated that although Sox2 may affect stomach patterning and cellular differentiation, as previously observed36,42, it does not appear to significantly alter stem cell maintenance or clonal fitness in the stomach epithelium.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41467-024-49382-y