Share this article
First-in-human trials are the initial stage of human clinical trials for a new drug or treatment. These trials involve testing the investigational product on healthy human subjects or patients with the target disease or condition. The primary objective of first-in-human trials is to evaluate the safety, dosage tolerance, absorption, metabolism, distribution, and elimination of the investigational product in humans. These trials also aim to identify any side effects associated with increasing doses and, if possible, gain early evidence of effectiveness. Industry-sponsored trials had more than 3.07 times more trials than non-industry-sponsored trials.
According to GlobalData’s Clinical Trials Database, the top countries for first-in-human studies include the US, UK, Australia, and Spain. When exploring the field of drug development, the US dominates first-in-human studies, as sites and institutions conduct a significant amount of research and have the infrastructure and resources to carry out these studies.
Additionally, the US has a robust regulatory framework that encourages and supports clinical research, including first-inhuman studies. Additionally, the US also has a diverse population, which allows for a more comprehensive understanding of how different individuals respond to different medications.
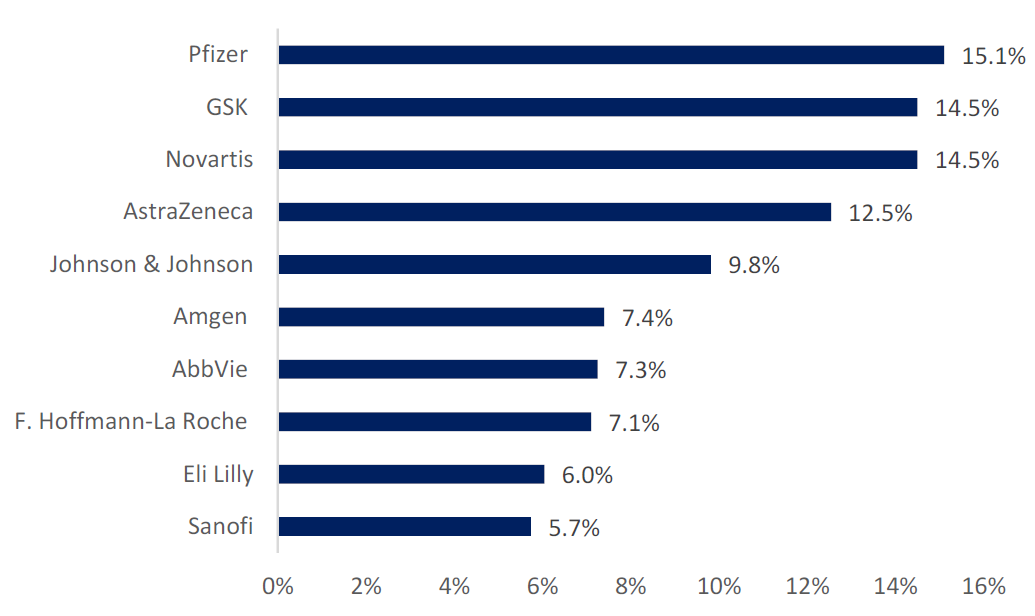
Oncology was the highest therapy area with 47% of trials, followed by infectious disease (13.4%) and central nervous system (10.6%). Top industry sponsors included Pfizer with 15% of trials, and then GSK and Novartis with 14% of trials each, as highlighted in Figure 1 (above).
Access the most comprehensive Company Profiles
on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Company Profile – free
sample
Your download email will arrive shortly
We are confident about the
unique
quality of our Company Profiles. However, we want you to make the most
beneficial
decision for your business, so we offer a free sample that you can download by
submitting the below form
By GlobalData
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.clinicaltrialsarena.com/analyst-comment/pfizer-leads-first-in-human-trials/
