Mice with the same genotype were assigned randomly to each group by an independent experimenter, but no specific randomization method was employed. Additionally, no statistical methods were utilized to pre-estimate the sample size. Experimenters responsible for histological analyses, IF and relevant laboratory tests were blinded to the grouping of the mice.
Animals
Male C57BL/6 J WT mice (6-8 weeks old) were provided by the Animal Center at the Nanfang Hospital of Southern Medical University (Guangzhou, China). Lgr5-EGFP-IRES-CreERT2 (Lgr5-GFP) mice were obtained from Jackson Laboratory (Ban Harbor, ME, USA). Breeding pairs of SOCS2 knocked-out mice were kindly provided by Zai-Long Chi (Wenzhou Medical University). SOCS2–/– mice in the C57BL/6 genetic background were generated by using the CRISPR/Cas9-mediated genome engineering technology. The deletion of the exon 3 fragment was carried out using gRNA1 (TTG GCA GTC GTT TTT CTA GT CGG) and gRNA2 (ATT CAG CTA AAA CTA CCT AA GGG) generated by Cyagen Biosciences (Guangzhou, China). All animals were housed in an animal room under specific pathogen-free (SPF) conditions (12 h light-dark cycle, temperature: 22 ± 1 °C and humidity: 55 ± 5%) and had ad libitum access to standard mouse chow (MD17121, MEDICIENCE, Jiangsu, China) and water. The animals used for the experiment were assigned randomly to the different groups. All animal procedures described here were approved by the local Animal Care and Use Committee of the Nanfang Hospital of Southern Medical University (Guangzhou, China) and carried out in line with the National Institutes of Health guidelines. All animal handling were according to the policy of the China Animal Welfare guidelines and under the supervision of the ethics committee of Southern Medical University. Mice were gavaged with 0.2 mL/20 g ddH2O or 250 mg/kg MA (M7397, Sigma-Aldrich, St. Louis, MO, USA) once a day consecutively for five days until surgery. The mice used here were randomized into the following experimental groups: (i) control; (ii) organoid transplantation; (iii) PBS liposome; (iv) clodronate liposome; (v) vehicle; and (vi) MA. Mice in the vehicle groups were gavaged 0.2 mL/20 g of ddH2O once a day for five days and used as the vehicle. Approval of this study was obtained from the Nanfang Hospital animal ethics committee (approval number: IACUC-LAC-20220508-001).
Establishment of the mouse intestinal I/R injury model
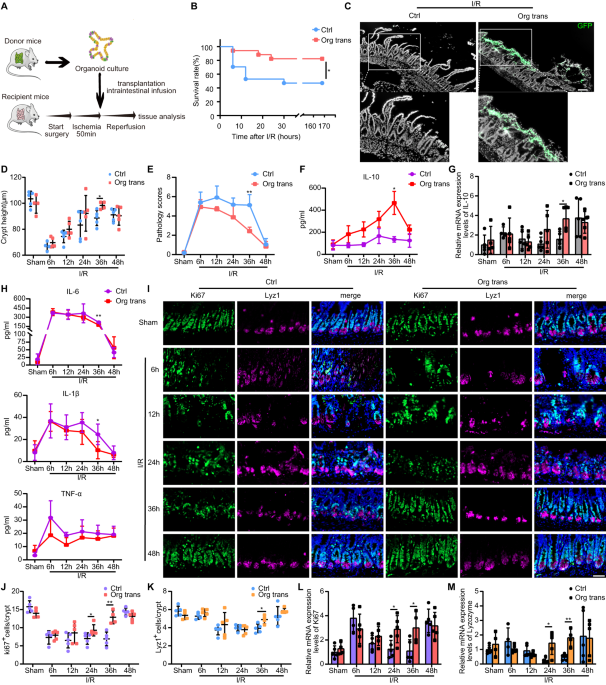
Before surgery, all animals were deprived of food for 18 h, but with ad libitum access to water. Mice were anesthetized using 2-3% inhalant isoflurane, and their skin was prepared before surgery. To establish the model, the small intestine was exposed through a mid-abdominal incision, and the superior mesenteric artery (SMA) was clipped for 50 min using a non-invasive microvascular artery clamp, followed by reperfusion, as previously described4. In brief, successful ischemia was identified through the pale color of the small intestine mucosa, while valid reperfusion was determined based on the reappearance of pink color. For liquid resuscitation, 1 mL of saline was immediately injected subcutaneously after the release of the clamp. After the abdominal incision was closed with suture, 1 mg/ml butorphanol (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) was injected subcutaneously for pain management. The survival of the mice was monitored every 6 hours after releasing the artery clamp and continued to be monitored for a study period of 7 days.
Culture of mouse intestinal organoids
Mice were sacrificed, the small intestine excised, immersed in sterile cold PBS, cut lengthwise, and rinsed using sterile cold PBS to wash away the intestinal contents. The intestine was then cut into pieces (2-4 mm) and placed in a 50-mL centrifuge tube which contained sterile cold PBS (prepared in advance), and a 2-ml pipette was used to wash the pieces via pipetting up and down repeatedly 15-20 times with sterile cold PBS. Then pieces were incubated for 15 min with 10 mL of 30 mM EDTA in PBS at room temperature. Subsequently, the reagent was removed, the intestinal sections were washed with PBS, and the supernatant fractions enriched in crypts were collected using a 70-µm cell strainer. These fractions were centrifuged at 400 × g for 5 min, following which the supernatant was removed. The precipitate was resuspended in 5 mL cold Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) solution (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), crypts were counted using an inverted microscope, and the required amount of liquid was aspirated into a centrifuge tube and centrifuged for 5 min at 400 × g. The precipitate was collected and resuspended in the same amount of organoid medium, including mouse epidermal growth factor (mEGF), Noggin, and R-spondin (ENR medium), and Matrigel (356231, BD Biosciences, Franklin Lakes, NJ, USA). The ENR medium contained DMEM/F12 media (Gibco), Primocin (100 µg/mL, InvivoGen, San Diego, CA, USA), N2 supplement (1X, Gibco), B27 supplement (1X, Gibco), mouse epidermal growth factor (50 ng/mL, PeproTech, Cranbury, NJ, USA), R-Spondin 1 (500 ng/mL, R&D Systems, Minneapolis, MN, USA), and murine Noggin (100 ng/mL, R&D Systems). Next, the suspension (50 µL) was quickly inoculated on preheated cell culture dishes to form a dome-shaped gelatinous structure. The dishes were then placed in a 37 °C incubator for 20 min. ENR medium was added to the culture dishes for organoid culture after the Matrigel was solidified.
Orthotopic transplantation
On transplantation day, freshly isolated crypts and sorted small-intestinal Lgr5+ stem cells were prepared as described in the methods. Mature mouse organoids were removed from the Matrigel and washed with cold DMEM/F12 solution. The isolated organoids, freshly isolated crypts and sorted small-intestinal Lgr5+ stem cells were then resuspended in Matrigel and DMEM/F12 (1:4) and kept on ice for no more than 30 min. Approximately 3×106 sorted small intestinal Lgr5+ stem cells, 8000 freshly isolated crypts, and 5000 organoids per 200 μL of fluid were obtained. Control DMEM/F12 and Matrigel were prepared under the same conditions but in the absence of organoids. Mice were anesthetized prior to induction of intestinal I/R injury. Immediately after the clips were released, 200 µL organoid solution was injected once into the duodenal lumen from the proximal to the distal portion of the small intestine using a 1-mL syringe with a 23-gauge needle and the control DMEM/F12 and Matrigel without organoids were injected in the same manner. Subsequently, the small intestine was carefully returned to the abdominal cavity, the incision was closed, and the anus was attached using tissue glue. Six hours after surgery, the glue was removed.
Blood sample and tissue processing
The mice were anesthetized before being sacrificed. Collection of blood samples was accomplished by removing the eyeballs, following which the blood samples were centrifuged for 15 min at 400 × g to collect sera. After the small intestines were removed, intestinal tissue was fixed using paraformaldehyde (PFA) for histopathological analysis. The lumen was then flushed with cold PBS, and the remaining tissue was collected and frozen using liquid nitrogen. After the cecum was excised, its contents were carefully squeezed out and harvested using sterile forceps. All collected samples were stored at −80 °C before use.
Fluorescence imaging
Prior to fluorescence detection, the entire small intestine was dissected from the mice. Fluorescence was detected using an Ami HTX Optical Imaging System (Spectral Instruments Imaging, Tucson, AZ, USA), and images were taken at 470 nm excitation, 570 nm emission, and 60 s exposure time using small binning (resolution). All images were analyzed and processed using ANALYSIS ONLY Aura 4.0.7 M.
Organoid-derived conditioned medium
Mouse intestinal organoids were cultured in six-well plates expanded for five to seven days. Then the organoids were cultured in the medium refreshed by DMEM/F12 in advance for 24 h, and the medium was collected and filtered through a 0.22 μm filter (431219, CORNING, Corning, NY, USA) after the 24 h culture to obtain the organoid-conditioned medium.
Histology and immunohistochemistry
For frozen-embedded sections, 4% PFA (Sigma-Aldrich) was used for the fixation of tissue and organoid samples, and 15% and 30% sucrose solutions were used to sequentially dehydrate samples, which were then embedded, frozen in OCT (Sakura Finetek), and sectioned at 6 μm intervals. For paraffin-embedded sections, 4% PFA was used to fix the samples, after that, samples were dehydrated using an ascending alcohol gradient, embedded in paraffin, and sectioned into 4 μm slices. Sample sections were then stained with hematoxylin and eosin (H & E). Evaluation of the degree of small intestinal injury was accomplished using Chiu’s method with some modifications64. In brief, the modification pathological scoring criteria are as follows: 0, normal intestinal mucosa and intestinal villus. 1, formation of Gruenhagen cavity begins at the tip of the intestinal villus. 2, formation of Gruenhagen cavity and slight damage of glands. 3, formation and enlargement of subepithelial gaps, congested and engorged capillaries. 4, moderate detachment of epithelium from lamina propria and gland damage. 5, partial loss of villus at the tip. 6, obvious loss of villus and dilated capillaries. 7, loss of lamina propria and significant gland damage. 8, beginning of digestion and decomposition of the lamina propria. 9, hemorrhage and formation of ulcers. The depth of the crypt was measured from the bottom to the opening. Paraffin-embedded sections were pressure-cooked in Tris-EDTA (pH 9.0) for 10 min before use. For permeabilization, cells and sections were incubated using 0.1% or 0.3% Triton X-100 (Thermo Fisher Scientific) in PBS at room temperature for 10 min and then washed and blocked in 10% goat serum (Gibco) at room temperature for 1 h. The primary and secondary antibodies used were diluted in 3% bovine serum albumin (BSA) in PBS. The primary antibodies that were used are as follows: anti-ZO-1 (1:400, ab216880, Abcam), anti-occludin (1:200, ab216327, Abcam), anti-Ki-67 (1:200, ab279653, Abcam), anti-OLFM4 (1:200, 39141 S, Cell Signaling Technology), anti-Muc2 (1:100, 27675-1-AP, Proteintech), anti-lysozyme (1:500, A0099, Dako) and anti-mannose receptor (1:200, ab64693, Abcam); DAPI (1:500, D9542, Sigma-Aldrich) was used to stain the nuclei. The positive cells were quantified in five randomly chosen crypts per image. Images were captured using an ortho fluorescence microscope (Axio Imager D2, Carl Zeiss), analyzed, and processed via ImageJ software (National Institutes of Health, Bethesda, MD, USA).
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
TRIzol reagent (10296028; Thermo Fisher Scientific) was used to extract the total RNA from the tissues, organoids, and cells. RNA purity and concentration were measured using a spectrophotometer (Nano Drop OneC; Thermo Fisher Scientific). Reverse transcription of RNA to complementary DNA (cDNA) was performed using a cDNA reverse transcription kit (FSQ-101, TOYOBO). Real-time PCR was performed using an ABI Q6 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), according to the SYBR Green detection protocol (QPK-201, TOYOBO). Finally, 18 S ribosomal RNA was used as a housekeeping gene, and the 2-ΔΔCT method was used to normalize data. Primers used here are listed (Supplementary Table 1).
Cytokine measurements
Cell culture medium and serum samples were thawed at room temperature. Next, the concentrations of IL-6, IL-1β, TNF-α, and IL-10 in the samples were measured using enzyme-linked immunosorbent assay (ELISA) kits (KE10002, KE10003, KE10007, and KE10008, Proteintech) in accordance with the manufacturer’s instructions.
Flow cytometry
The small intestine was washed using PBS, and Peyer’s patches and fat tissue were removed47. The intestines were opened lengthwise, cut into pieces and incubated with 10 mL, 5 mM EDTA for 15 min, and the supernatant containing epithelial cells was discarded. The remaining tissues were transferred into RPMI-1640 medium containing collagenase IV (0.5 mg/mL, C5138, Sigma-Aldrich), DNase I (100 U, 10104159001, Roche), and incubated at 37 °C for 1 h. Subsequently, the supernatants were discarded, the tissue fragments were washed repeatedly with PBS, and the supernatants containing laminar cells were collected. The fractions were resuspended in Percoll (GE17-0891-01, Sigma-Aldrich) after centrifugation, and the middle interface layer was harvested after density gradient centrifugation. The cell precipitate was resuspended for subsequent use. Small intestinal macrophages were cultured in 10% fetal bovine serum (FBS) and stained with APC-Cy7–conjugated anti-CD45 (I3/2.3, A15395, Thermo Fisher Scientific), BV510-conjugated anti-mouse CD11b antibody (clone M1/70, 101263, Biolegend), FITC-conjugated mouse F4/80 (clone BM8, 123108, Biolegend), and sorted.
Spleen tissues were carefully pulverized using a 70-μm cell strainer, rinsed in PBS, collected in 15-mL centrifuge tubes, and centrifuged at 400 × g for 5 min. The precipitate was resuspended in erythrocyte lysis buffer (Miltenyi Biotec, Bergisch Gladbach, Germany) and incubated for 5 min on ice. Subsequently, digestion was terminated using cold PBS and the sample was centrifuged at 400 × g for 5 min. The remaining cell pellets were resuspended within cold PBS and stored at 4 °C until further use.
After rinsing the bone marrow tissues derived from bone marrow cavities with DMEM (Gibco), the suspensions were centrifuged. Red blood cells were lysed using lysis buffer and resuspended in cold PBS for later use.
Blood samples were centrifuged at 400 × g for 15 min to obtain sera, and the remaining samples were diluted with 1 mL PBS. The diluted blood samples were then transferred gently into 15-mL centrifuge tubes containing 1 mL Ficoll Histopaque (P8900, Solarbio Life Sciences, Beijing, China). This led to the formation of two distinct layers, which were then centrifuged for 15 min at density gradients. The cell precipitate was resuspended for testing.
The cells were incubated with a protein transport inhibitor cocktail (554724, BD Biosciences) at 37 °C for 4 h and Fc block (553141, BD Pharmingen, Franklin Lakes, NJ, USA) at 4 °C for 15 min. The cells were then incubated at 4 °C for 30 min in the dark for surface staining with fluorophore-conjugated antibodies before being fixed, permeabilized, and incubated with intracellular cytokine staining antibodies against IL-10 (clone JES5-16E3) and CD206 (clone C068C2). Flow cytometry acquisition was performed using an LSRFortessa X-20 Multidimensional HD Flow Cytometer (BD Biosciences), while data analysis was performed using FlowJo software (Tree Star Inc., Ashland, Or, USA). The antibodies used are listed in Supplementary Table 2.
Small intestinal crypts were isolated from Lgr5-EGFP-IRES-CreERT2 mice, as described above. Crypts were further dissociated into single cells using TrypLE Express (Invitrogen, Waltham, MA, USA). Enhanced GFP (EGFP) stem cells were gated and sorted using a MoFlo XDP ultra-speed flow cell sorting system (Beckman Coulter, Brea, CA, USA).
Depletion of intestinal macrophages
Two hundred microliters of clodronate- or PBS-loaded liposomes (CP-005-005, LIPOSOMA, Groningen, The Netherlands) were intravenously injected into mice thrice on alternating days prior to intestinal I/R to deplete intestinal macrophages.
Participants
Twenty-three participants undergoing coronary artery bypass graft or elective cardiac valve replacement surgery, at the cardiac surgery department of the Nanfang Hospital of Southern Medical University (Guangzhou, China), were consecutively recruited between September 2020 and November 2021. All participants were aged between 18 and 75 years. Exclusion criteria included those with chronic digestive system diseases, previous gastrointestinal surgery, chronic kidney disease, confirmed or suspected intestinal ischemia/necrosis, and those who had used prebiotics, laxatives or antidiarrheals within one week, or antibiotics within three months before the start of the study. No significant differences between the demographic and health-related characteristics of the groups were observed. Written informed consent was obtained from all patients. Ethical committee approval of this study was obtained from the Nanfang Hospital of Southern Medical University (approval number: NFEC-202009-k2-01).
Lausanne intestinal failure estimation (LIFE) score
The gastrointestinal failure score of patients was calculated on the seventh day after surgery in accordance with LIFE score estimation, as described previously65. Briefly, the evaluation criteria encompass various parameters, including levels of intra-abdominal pressure, lactate, gastric residue, enteral nutrition, motility, and bowel sounds.
Human FABP2/I-FABP immunoassay
Human blood samples were collected preoperatively and 12 h after surgery using an anticoagulant (EDTA or heparin) and centrifuged at 400 × g for 15 min to separate the upper layer of plasma. All samples were stored at −80 °C before use. Human plasma FABP2/I-FABP levels were determined using a human FABP2/I-FABP Quantikine ELISA kit (DFBP20, R&D Systems) following the manufacturer’s instructions, researchers being blinded to group allocations.
D-lactate assay kit
Frozen human plasma samples were thawed at room temperature before detection. In accordance with the manufacturer’s protocol, the colorimetric D-lactate assay kit (ab83429, Abcam) was used to measure D-lactate concentration in the plasma by researchers who were blinded to the group allocations.
BMDM culture
To isolate BMDMs, the femur and tibiae of 7-8 week-old C57BL/6 J mice were flushed, the suspension centrifuged at 400 × g for 10 min and the supernatant discarded. Subsequently, the precipitate was resuspended in DMEM (Gibco) supplemented with 10% FBS (Gibco) as well as 20% L929 conditioned mediam and cultured in a 37 °C cell incubator. Seven days after differentiation, the cells were harvested for experiments. In the conditioned medium experiment, cells were cultured in organoid-derived conditioned medium or DMEM/F12 as a control. In the MA stimulation experiment, cells were cultured with 4 μM MA or an equal volume of double-distilled water (ddH2O) as a vehicle. Cells treated with siRNA for 24 h before stimulation with MA were used as the negative control (NC) group. Cells were randomized into different groups as follows: (i) vehicle; (ii) MA; (iii) vehicle + siNC; (iv) vehicle+ siSOCS2; (v) MA + siNC and (vi) MA + siSOCS2.
Untargeted and targeted metabolomics
Untargeted metabolomic analysis was performed by a Vanquish UHPLC system (Thermo Fisher Scientific) and an Orbitrap Q ExactiveTMHF-X mass spectrometer (Thermo Fisher Scientific). To obtain extracts, samples were homogenized and repeatedly centrifuged, and the final fecal extracts were aliquoted for metabolite profiling analyses. Raw data was generated using ultra-high performance liquid chromatography-mass spectrometry and processed by Compound Discoverer 3.1 (CD3.1, Thermo Fisher Scientific) for integration, normalization, and peak intensity alignment. Next, mzCloud, mzVault, and MassList databases were used to match the processed dataset. Principal component analysis and OPLS-DA were performed using normalized data, and VIP > 1 was considered as the threshold.
Targeted quantitative metabolomics was conducted using TSQ Quantiva™ (Thermo Fisher Scientific). The MA standard (L46691, Acmec, Biochemical, Shanghai, China), αKG standard (61234, Sigma-Aldrich) were dissolved in 4:1 methanol/water (v/v) to prepare the standard curve. The samples were homogenized with 80% methanol, sonicated for 3 min, and centrifuged at 14,000 × g at 4 °C for 10 min. The supernatant obtained was filtered for subsequent experiments. A Prelude SPLC™ sample preparation and liquid phase system (Thermo Fisher Scientific) was used to carry out Chromatographic separation, TSQ Quantiva (Thermo Fisher Scientific) was used to perform quantitative detection and the TraceFinder™ software version 3.3 SP1 (Thermo Fisher Scientific) was used to complete data collection.
RNA-seq
RNA was extracted as mentioned above. RNA sequencing was performed by Shanghai Biotechnology Corporation. Briefly, all RNA samples underwent quality control analysis using Fast QC (version 0.10.1). Sequencing libraries were prepared using a TruSeq Stranded Total RNA Library Prep Kit (15032612, Illumina, San Diego, CA, USA). CBot (HiSeq2500) was used to generate clusters and the HiSeq2500 sequencing platform (Illumina) was used to generate pair-end 150 bp reads. Valid clean data were obtained using Trimadap to filter out unqualified sequences, while HISAT2 was used for reference genome alignment. Based on HISAT2 alignment results, transcripts were reconstructed using StringTie (John Hopkins University, Baltimore, MD, USA), and expression levels of all genes in each sample were calculated. The computation of differential gene expression was completed by the negative binomial model implemented in the Bioconductor package, edgeR. A fold change (FC) ≥ 2 or FC ≤ -2 with a q-value < 0.05 was considered significant.
siRNA transfection
Mouse siRNA targeting SOCS2 and negative control siRNA were designed and constructed by Ribo Targets (Guangzhou, China). Transfection was performed via Lipofectamine 3000 (L3000015, Invitrogen), following the manufacturer’s instructions. The efficiency of SOCS2 knockdown was assessed using qRT-PCR and western blotting.
Macrophage isolation
Donor mice were gavaged with MA for 5 consecutive days and subjected to intestinal I/R injury for 36 h. Then the mice were sacrificed and the spleen was harvested. Spleen immune cell was isolated as described above and macrophages were further isolated by using magnetic bead separation methods. In short, determine the cell number in the single cell suspension and centrifuge cell suspension. Incubate the cell pellet with anti-F4/80 microbeads (130-110-443, Miltenyi Biotec, Germany) according to the manufacturer’s instructions.
Adoptive transfer
Recipient mice underwent intestinal I/R injury were injected intravenously with 2-3 × 10^6 macrophages immediately after the clamp was released.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 8.3.0) software. Two-tailed log-rank test was used for the Kaplan-Meier survival curve. The Shapiro-wilk test is used to determine whether the data is normally distributed. For normally distributed data, two-tailed student’s t test was used to compare the means of two independent groups. One-way ANOVA and Two-way ANOVA, followed by Tukey’s multiple comparisons test, were used to compare the means of more than two groups. For non-normally distributed data, the nonparametric method such as Mann–Whitney test was used. All data are expressed as mean ± standard deviation. P < 0.05 was considered statistically significant. For further statistical details, refer to individual figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41467-023-42502-0
