Design and creation of opto-caspase8
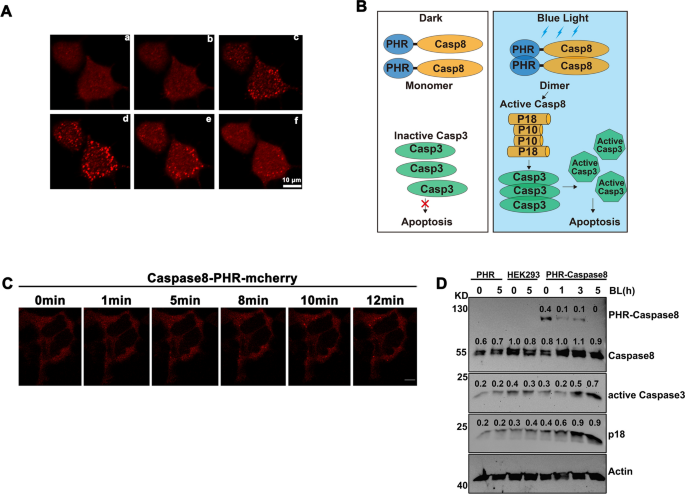
In previous studies, it has been found that CRY2 or its PHR domain (CRY2PHR) underwent oligomerization in a blue light dependent manner16,17,18. To confirm CRY2PHR can underwent oligomerization in human cells, we fused PHR to mcherry and transfected into HEK293T cells, and we found that PHR-mcherry can cluster in a blue light dependent manner (Fig. 1A) as expected. To control the caspase8 mediated signaling pathway with blue light, we first fused the PHR domain of CRY2 with caspase8 (PHR-Caspase8) as the new optogenetic tool (Opto-Casp8-V1). As illustrated in Fig. 1B, the PHR-Caspase8 was monomer and kept inactive in darkness. Under blue light irradiation, the PHR-Caspase8 dimerized and oligomerized because of the blue light specific oligomerization of PHR. The oligomerized PHR-Caspase8 then self-cleavaged and released the activated caspase8 domain (P18 and P10) to active downstream caspase3 and promote cell apoptosis. To further confirm the ability of the fusion protein to aggregate, we assessed the aggregation of caspase-PHR-mCherry in HEK293T cells following blue light induction. The results demonstrated that it can indeed aggregate (Fig. 1C). To confirm this process, we transferred the constructed Opto-Casp8-V1 (PHR-Caspase8) including the PHR-only and empty vector control into HEK293T cells, respectively, and treated them with blue light for 0, 1, 3 and 5 h respectively, lysed the cells, and detected the protein level by western. We analyzed the status of PHR-Caspase8, the activated P18 and the downstream protein from dark to blue light irradiation. As excepted, the abundance of the precursor PHR-Caspase8 was decreased after blue light irradiation (Fig. 1D), which suggested the PHR-Caspase8 could self-cleavage and consume in blue light driven by the oligomerization of PHR. On the other hand, the activated P18 of caspase8 and the activated caspase3 were accumulated from dark to blue light irradiation (Fig. 1D), suggesting that the activity of PHR-Caspase8 could be controlled by blue light to active the downstream signaling pathway in blue light.
Design of optogenetic tools to control cell apoptosis by blue light. (A) Blue light induces CRY2-PHR-mcherry oligomerization in HEK293T cells. a. dark; b. blue light 1 min; c. blue light 5 min; d. blue light 10 min; e. blue to dark 5 min; e. blue to dark 10 min. (B) The photo-responsive region of Arabidopsis thaliana cryptochrome 2 (PHR, amino acid 1–498) is fused with Caspase8. In the dark, the engineered PHR-Casp8 exhibits monomer. Upon blue light illumination, PHR drives dimerization of PHR-Casp8 and promoters the apoptosis. Casp8, Caspase8; Casp3, Caspase3; PHR, the N-terminal domain of CRY2. (C) Clustering of caspase-8-PHR-mCherry in response to blue light in HEK293T cells, bar = 10 μm. (D) Blue light actives Opto-Caspase8-V1 in HEK293 T cells. Transfected HEK293T cells were crushed by Pierce IP lysis buffer, total cell lysates were analyzed by western blotting probed with anti-Caspase8, anti-Caspase3, actin was used as a loading control.
Blue light induce opto-caspase8 cluster and self-cleavage
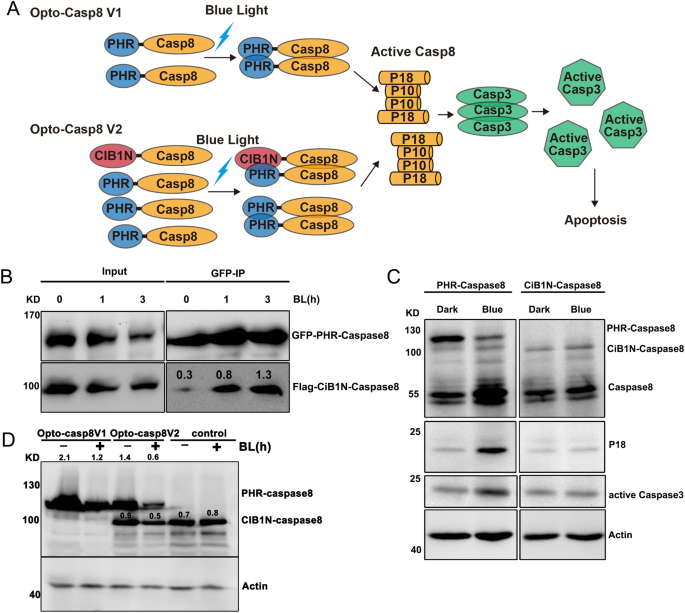
Building upon earlier findings that the Arabidopsis blue light receptor CRY2 can interact with the transcription factor CIB1 in a blue light-dependent manner, we created an optogenetic tool for caspase8 by fusing the N-terminal domain of CIB1 (amino acids 1–170) with caspase8 to generate CIB1N-caspase8, which was then combined with PHR-caspase8 to produce Opto-Casp8-V2 (Fig. 2A). This design ensured that the optogenetic system could effectively regulate the caspase8-mediated signaling pathway via blue light. To validate this approach, we transfected HEK293T cells with GFP-PHR-caspase8/Flag-CIB1N-caspase8 (Opto-Casp8-V2 cassette), treated the cells with blue light, and subsequently lysed them. We performed immunoprecipitation (IP) with GFP trap and observed that all the proteins of GFP-PHR-caspase8/Flag-CIB1N-caspase8 underwent blue light-dependent cleavage and interacted in a blue light-enhanced manner in co-IP (Fig. 2B). These results indicate that Opto-Casp8-V2 can effectively function in cells, similar to Opto-Casp8-V1.
Blue light trigger Opto-Caspase8 cleavage and activation. (A) N-terminal part of cryptochrome interacting basic-helix–loop–helix protein CIB1 (amino acids 1–170, CIB1N) are fused with Caspase8, PHR-Caspase8 is marked as Opto-Casp8 V1 and PHR-Caspase8/CiB1N-Caspase8 as Opto-Casp8 V2. CIB1N, the N-terminal domain of CIB1. (B) Co-IP assay showed blue light enhanced the interaction of Opto-Caspase8 V2 in HEK293 T. Transfected HEK293T cells were treated with blue light (30 μmol m−2 s−1) for indicated time before lysed. The immunoprecipitation signals were probed by anti-GFP or anti-Flag, respectively. (C) Blue light actives Opto-Caspase8 V1 in HeLa cells. (D) Cleavage efficiency of caspase8 between Opto-Caspase8 V1 and Opto-Caspase8 V2 in Hela cells.
We next evaluated the effectiveness of PHR-caspase8 and CIB1N-caspase8 in the human cervical cancer cell line HeLa. We firstly transfected HeLa cells with PHR-caspase8 and CIB1N-caspase8, treated them with blue light, and observed that PHR-caspase8 exhibited highly efficient self-cleavage and induced downstream accumulation of caspase3, whereas CIB1N-caspase8 could not be activated by blue light (Fig. 2C). We compared the cleavage efficiency mediated by Opto-Casp8-V1 and Opto-Casp8-V2 in HeLa cells and found that Opto-Casp8-V2 demonstrated significantly more effective self-cleavage and consumption than Opto-Casp8-V1 under blue light, indicating that CIB1N-caspase8 could enhance the activation of PHR-caspase8 in the Opto-Casp8-V2 optogenetics cassette (Fig. 2D). This finding further suggests that, in addition to the oligomerization of CRY2, the CRY2-CIB1 protein–protein interaction can further promote the activation of precursor caspase8 in blue light.
Optogenetic control of caspase8-mediated apoptosis and programmed cell death
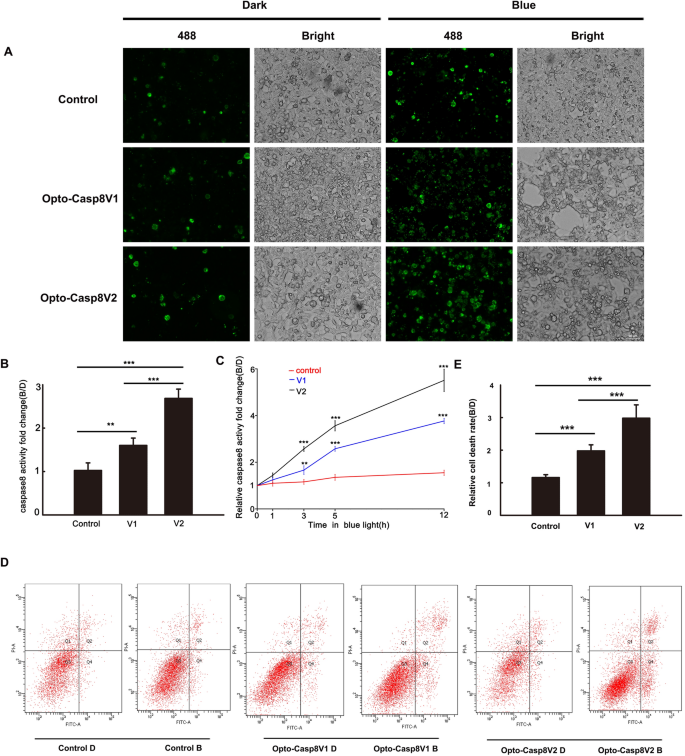
We utilized FAM-LETD-FMK caspase-8 to evaluate the effectiveness of caspase-8 activation in live cells using our Caspase8 optogenetic tool. Plasmids encoding Opto-Caspase8-V1 and Opto-Casp8-V2 were transfected into HEK293T cells, and after blue light stimulation, the cells were labeled with FAM-LETD-FMK and imaged using an inverted microscope (Zeiss Axio Observer A1). Our results demonstrated that Opto-Casp8-V1 and Opto-Casp8-V2 induced greater activation of caspase-8 in live cells than cells transfected with the CIB1N-Caspase8 control (Fig. 3A,B). Moreover, Opto-Casp8-V2 exhibited significantly higher activation efficiency than Opto-Casp8-V1 (Fig. 3A,B), confirming the usefulness of CIB1N-Caspase8 as an optogenetic tool for caspase8 signaling pathway activation. We further examined the morphological changes in cells transfected with Opto-Casp8-V1 and Opto-Casp8-V2, and our data showed that Opto-Casp8 induced more active Caspase8 in HEK293T cells after effective self-cleavage under blue light, resulting in apoptosis (Fig. 3A,B). A time-course assay of FAM-LETD-FMK caspase-8 activity demonstrated a positive correlation between blue light treatment duration and FAM-LETD-FMK caspase-8 activity (Fig. 3C), further supporting the ability of Opto-Casp8 to trigger apoptosis in human cells.
Blue light dependent apoptosis activated by Opto-Caspase8. (A) Active caspase8 was detected by fluorescence microscope labeled by FAM-LETD-FMK caspase-8 in HEK293T cells. Blue light (50 μmol m−2 s−1 for 3 h) treated transfected cells were incubated with FAM-LETD-FMK caspase-8 before capturing pictures, CiB1N-Caspase8 and the empty vector pci myc were co-transfected as control. (B) Caspase8 activity analysis in (A), activities = fluorescent intensity of caspase8 in blue/fluorescent intensity of caspase8 in dark. Data are presented as mean ± SD (n = 3). Student’s t test: ***p < 0.001. (C) Time course of FAM-LETD-FMK labeled caspase-8 activity assay in HEK293T cells. Same operation were conducted as showed in (A,B). Data are presented as mean ± SD (n = 3). Student’s t test: ***p < 0.001. (D) Cell viability analysis in HeLa cells expressed Opto-Caspase8 V1 or Opto-Caspase8 V2 by flow cytometry. Alexa Fluor® 488 annexin and PI was used to detect apoptosis cells. The transfected cells were either shielded or exposed to continuous blue light (50 μmol m−2 s−1) for 3 h. (E) Relative cell death rate analysis in (D). Rate = number of apoptosis cells in blue/number of apoptosis cells in dark. Data are presented as mean ± SD (n = 3). Student’s t test: ***p < 0.001.
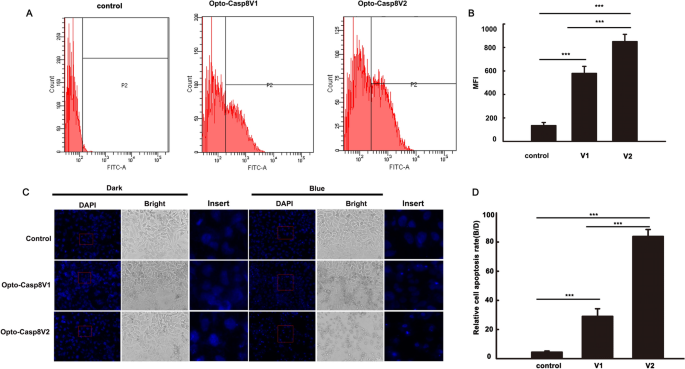
To investigate the efficacy of Opto-Casp8-V1 and Opto-Casp8-V2 in inducing cell apoptosis via the caspase8-mediated signaling pathway under blue light, we employed flow cytometry to monitor apoptosis in cells induced by Opto-Casp8-V1 and Opto-Casp8-V2. During apoptosis, phosphatidylserine (PS) flips to the outer layer of the lipid membrane19,20. Annexin-V is a Ca2+-dependent phospholipid-binding protein with a molecular weight of 35–36 kD, which exhibits high affinity for phosphatidylserine21. The combination of phosphatidylserine and Annexin-V exposed on the lateral side of the cell is indicative of cell apoptosis22. Therefore, we used Alexa Fluor® 488 Annexin-V/PI with flow cytometry to verify cell apoptosis. Plasmids encoding Opto-Casp8-V1 and Opto-Casp8-V2 were transfected into HeLa cells, and after blue light stimulation, Alexa Fluor® 488 Annexin-V and PI-labeled cells were detected by flow cytometry. Our results indicated that compared to the control group (CIB1N-Caspase8), Opto-Casp8-V1 and Opto-Casp8-V2 significantly promoted cell apoptosis under blue light (Fig. 3D). Additionally, Opto-Casp8-V2 exhibited a stronger effect on promoting cell apoptosis than Opto-Casp8-V1 (Fig. 3D,E), which is consistent with the previous results of cleavage efficiency. Similarly, we employed flow cytometry to assess caspase-8 activity in HeLa cells following transfection with Opto-Casp8-V1 and Opto-Casp8-V2 plasmids. The results revealed that, after transfection with Opto-Casp8-V2, its activity was significantly higher than Opto-Casp8-V1, and both Opto-Casp8-V1 and Opto-Casp8-V2 activities were noticeably higher than the control (Fig. 4A,B). This indicates that Opto-Casp8-V1 and Opto-Casp8-V2 are capable of inducing cell apoptosis in HeLa cells.
Opto-Caspase8 cause cell death in HeLa cells. (A) Detecting the caspase-8 activity with FAM-LETD-FMK with flow cytometry in HeLa cells under blue light, the empty vector was transfected as control. (B) Mean fluorescence intensity (NFI) of (A). Data are presented as mean ± SD (n = 3). Student’s t test: ***p < 0.001. (C) The transfected cells were either shielded or exposed to pulse blue light (50 μmol m−2 s−1) for 12 h and incubated with DAPI, after washing for 3 times, pictures were obtained by Zeiss Axio Observer A1 microscope, CiB1N-Caspase8 and the empty vector pci myc were co-transfected as control. (D) Relative cell apoptosis rate analysis of (A), rate = number of condensed nuclei in blue/number of condensed nuclei in dark. Data are presented as mean ± SD (n = 3). Student’s t test: ***p < 0.001.
The process of apoptosis involves multiple stages of morphological changes. In the initial stage, chromatin condenses and separates, distributing along the nuclear membrane. The cytoplasm also undergoes shrinkage, but its membrane remains intact with selective permeability. In the late stage of apoptosis, the chromatin breaks into fragments of varying sizes, which aggregate with organelles such as mitochondria and are surrounded by the inverted cell membrane. Subsequently, they gradually separate to form condensed nuclei23,24. In light of these observations, we employed DAPI nuclear staining to detect programmed cell death induced by Opto-Casp8. Our results demonstrated that Opto-Casp8 V1 and Opto-Casp8-V2 triggered the shrinkage of a large number of nuclei to form condensed nuclei, ultimately promoting cell death (Fig. 4C,D). In summary, our optogenetic tools (Opto-Casp8-V1 and Opto-Casp8-V2) exhibit caspase-8 activation activity in a blue light-dependent manner, subsequently activating downstream proteins to control cell apoptosis with blue light.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-023-50561-y