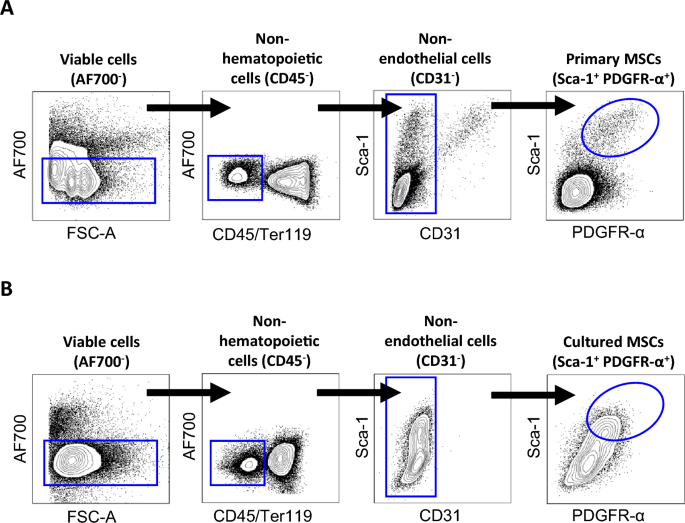
Over the years, extensive research has established the role of MSCs in diseases such as cancer20,21. MSC-based regenerative therapies have been trialled and implemented for a range of injuries and illnesses11,22. However, the rarity of this stem cell population necessitates its expansion in culture prior to clinical use. The most commonly used passages for transplantation are from passage 3 to 7, however many studies have found that the passaging process inadvertently alters MSC biology and function13,14,15,16,17,18. To date, changes identified by culturing MSCs at increasing passages include alterations to DNA damage response, senescence, multipotent potential and immunomodulatory properties13,14,15,16,17,18. Others have shown that an increase in the passage number does not significantly impact the immunoprivilege of MSCs23. However, no study has directly compared changes between primary MSCs and MSCs after one passage. Here we provide unique insight into culture-induced molecular changes in MSCs following just a single passage.
Previous studies have demonstrated that MSCs have limited proliferative potential in vitro and undergo senescence with increasing cell passages13,15. However, it should be noted that these studies examined the biological aging of MSCs when the cells were already under maintenance in an in vitro setting. In contrast, our study explored the molecular impact of cell culture on MSCs using primary MSCs and cultured MSCs. In fact, we observed a clear distinction in the molecular signatures of cultured MSCs and primary MSCs, particularly in upregulation of genes involved in ‘cell cycle’, ‘cell division’ and ‘DNA replication’ in cultured MSCs, suggesting that MSC turnover is increased following early passage in vitro. Our results identified that just 10 days in culture induced the upregulation of genes associated with cell cycling. Ccna2 and Anln are responsible for cellular proliferation and were the most significantly upregulated genes associated with ‘cell cycle’ and ‘cell division’ biological processes24,25. In support of this, we also found that the number of the cultured MSCs after 10 days in culture (average of 1,03,250 cells per mouse) was approximately 27-fold higher than the number of the primary MSCs (average of 3850 cells per mouse). Further studies should be conducted to further investigate the mechanistic role of these genes in this setting and exploit the strategy of genetically manipulating Ccna2 and Anln in vitro to overcome MSC senescence with increasing cell passages. In addition, western blot analysis could also be conducted to evaluate changes in the signaling molecules associated with cell cycle and cell division, such as cyclins, PCNA and Ki67.
Another important characteristic of MSCs is their trilineage differentiation potential. Whilst we previously confirmed that cultured MSCs can differentiate into osteoblasts, adipocytes and chondrocytes, our transcriptomic analyses suggest that differentiation potential may vary between primary MSCs and cultured MSCs26. For example, GO enrichment analysis identified that genes associated with ‘osteoblast differentiation’, ‘ossification’ and ‘bone mineralization’ were downregulated in cultured MSCs. A reduction in MSC osteogenic potential has previously been noted following long-term culture15,17. However, our data provide striking evidence that the osteogenic potential of MSCs may be compromised as early as following a single passage in culture. Interestingly, one study has successfully demonstrated the feasibility of preconditioning MSCs with cytokines (e.g., tumor necrosis factor-alpha) to promote osteogenesis27. These findings are clinically important as MSCs are being trialled for their potential to regenerate bone in human subjects28.
GO enrichment analysis also identified changes to the adipogenic- and chondrogenic-lineage potentials of MSCs following culture. While contention remains regarding the effect of long-term culture on the adipogenic potential of MSCs, our data suggest that culturing MSCs could potentially impair adipogenesis after just one passage15,17,29. Furthermore, genes classified as negative regulators of chondrocyte differentiation were downregulated in cultured MSCs, indicating a possible differentiation bias towards the chondrocyte lineage. However, others have reported reduced chondrogenic potential in MSCs following long-term culture, indicating that any bias towards the chondrocyte lineage may only be short-lived29. Future studies could validate the effect of cell culture on the differentiation potential of primary MSCs by performing tri-lineage differentiation assays. In addition, the self-renewal capacity of MSCs could also be investigated by comparing the colony-forming unit ability of primary MSCs and cultured MSCs.
MSCs are also capable of regulating the proliferation and activation of immune cells, thereby modulating the body’s response to injury and illness30,31. Many studies are currently exploring the role of MSC immunomodulation in disease and for the treatment of immune disorders32. However, long-term culture can impair the immunosuppressive capabilities of MSCs18. Corroborating these findings, we identified that genes downregulated in cultured MSCs were enriched for biological processes including ‘response to bacterium’, ‘immune response’ and ‘complement activation’, indicating that impairment of the immunomodulatory ability of MSCs may be altered after just 10 days in culture. For example, a gene found to be downregulated in cultured MSCs was Enpp2, which encodes for autotaxin. Autotaxin is known to mediate lymphocyte migration, thus supporting the proposition that its downregulation may impair MSC immunomodulation33. Future studies could validate the immune modulatory properties of primary MSCs and cultured MSCs by priming these cells with cytokines such as IFN-γ, TNF-α, and IL-1β, and subsequently assess changes in the production/secretion of functional factors that regulate immune responses.
Interestingly, the impact of cell culture on the molecular signature of MSCs have also been demonstrated in several studies involving the use of MSCs derived from human bone marrow. For instance, genes involved in regulation of the complement cascade (SERPING1), antitumoral immunity (OAS2) and osteogenesis (ALPL) were found to be downregulated in cultured adherent human bone marrow-derived MSCs compared to primary human bone marrow-derived MSCs34. In addition, separate studies have shown that uncultured human bone marrow-derived CD45lowCD271+ MSCs demonstrate distinct transcriptional differences compared to cultured bone marrow-derived MSCs35,36. These data from primary human bone marrow MSCs corroborate our findings from murine bone-derived MSCs, supporting the impact of culture-induced molecular changes on MSCs.
Another area of interest is the impact of culture methodologies on MSC biology. Unlike human bone marrow, murine MSCs are not easily isolated from bone marrow aspirates by plastic adherence due to contaminating hematopietic cells. However, the discovery that MSCs are found lining the inner surface of compact bones has given rise to a new method where murine MSCs can be directly isolated from bones cleared of bone marrow, including one pioneered by Zhu et al. where MSCs were successfully isolated from collagenase II-digested bone fragments in vitro37. Although this method can remove contaminating hematopoietic cells, it is worth noting that this would involve multiple cell passages which could inadvertently affect MSC biology. Therefore, this necessitates further protocol refinements in the future. In addition, several studies have examined the influence of technical variables including culture medium, dynamic culture, 3-dimensional (3D) culture and oxygenation on MSC biology38,39,40,41,42,43,44,45. For instance, a previous study has shown that high levels of oxygen exposure can alter multilineage differentiation capacity of primary bone marrow-derived mouse MSCs, as well as inducing cellular stress, growth arrest and apoptosis via p53 activation46. Whilst our study did not specifically examine whether atmospheric oxygen can affect the physiology of long bone-derived MSCs and p53 activity, this remains an avenue for future investigation. Remarkably, dynamic culture systems, preconditioning with cytokines and the use of 3D scaffolds has been found to improve the osteogenic differentiation potential and/or immunomodulatory capabilities of cultured MSCs, which highlights the importance of optimizing culture methodology41,47,48,49. It is also worth noting that there is a lack of consensus with regards to defining MSCs based on surface markers. For instance, some studies define populations of MSCs based on the expression of LEPR and Nestin, while we defined our bone-derived MSCs based on Sca-1 and PDGFRα19,50,51. Therefore, consensus regarding a standardized protocol for MSC definition and culture will be beneficial for stem cell research going forward.
A key limitation of our current study is the difficulty of obtaining high numbers of primary MSCs for bulk RNA sequencing via fluorescence-activated cell sorting (FACS) due to the rarity of this population. While we managed to obtain a total of four cultured MSC samples from culturing heterogenous bone cells prior to FACS, we sorted and pooled primary MSCs from four mice and six mice respectively, which only yielded two biological samples for the primary MSC group. Future studies could utilize next-generation sequencing techniques, such as single cell RNA sequencing to overcome this limitation. It is also important to note that our study can only delineate the molecular mechanisms, rather than biological properties using conventional assays due to the fundamental difference between in situ-derived primary MSCs and in vitro-derived cultured MSCs. These differences may also be reflected by the notion that MSCs reside in BM niches where crosstalk among MSCs and niche cells influence their biology, in contrast to mono-cultured MSCs which are deprived of this critical influence. Preclinical experiments which utilize cultured MSCs may therefore not accurately recapitulate in vivo phenotypes and thus consideration of this caveat should be made when interpreting results.
In conclusion, this study provides insight into the molecular changes of murine MSCs after a single passage in culture. Our findings provide a foundation from which we can optimize MSC culture methodology to maintain the characteristics of primary MSCs and investigate the impact of culture-induced changes on the therapeutic efficacy of MSCs in the clinic.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-024-63009-8
