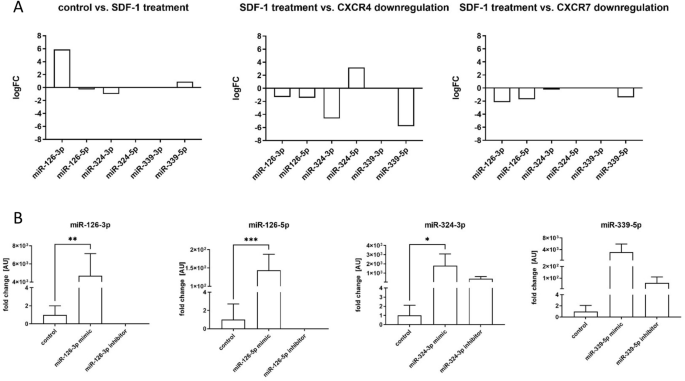
Changes in miRNA level after SDF-1 treatment or Cxcr4 or Cxcr7 down-regulation
In our previous study, we noticed that SDF-1 impacts SC-derived myoblasts migration and participation in skeletal muscles regeneration6, 8,9,10, 23. However, little is known about the impact of miRNA on SDF-1 signaling. To select miRNAs engaged in SDF-1 regulation the next generation sequencing (NGS) was performed using control, SDF-1 treated, SDF-1 treated and Cxcr4 siRNA or SDF-1 treated and Cxcr7 siRNA transfected SC-derived myoblasts. NGS allowed us to analyze the level of microRNA which expression changes in control and treated cells. We selected 6 molecules: miR-126a-3p, miR-126a-5p, miR-324-3p, miR-324-3p-5p, miR-339-5p-3p, miR-339-5p-5p that level differed significantly between control SC-derived myoblasts, those treated with SDF-1, and those in which Cxcr4 or Cxcr7 were down-regulated (Fig. 1A). The level of miR-126a-3p and miR-339-5p-5p increased in SDF-1 treated SC-derived myoblasts, compared to control ones. On the other hand, the level of miR-126a-3p, miR-126-5p, miR-324-3p, and miR-339-5p-5p decreased in SC-derived myoblasts in which Cxcr4 or Cxcr7 were downregulated, if compared to those treated with SDF-1. Thus, SC-derived myoblasts were transfected with miRNA126-3p, miR-126-5p, miR-324-3p, miR-339-5p mimics or selected microRNA inhibitors to follow changes in cell proliferation, migration, differentiation, and transcriptome. First, we confirmed that transfection with specific microRNA mimics or inhibitors, respectively, led to up-regulation or downregulation of specific miRNAs in SC-derived myoblasts (Fig. 1B).
Analyzes of miRNA level and transfection efficiency of mouse SC-derived myoblasts; (A) The changes of the levels of miR-126-3p, miR-126-5p, miR-324-3p, miR-324-5p, miR-339-3p, and miR-339-5p in SC-derived myoblasts after SDF-1 treatment, down-regulation of CXCR4 or down-regulation of CXCR7 assessed by next generation sequencing; (B) Expression of miR-126-3p, miR-126-5p, miR-324-3p, and miR-339-5p in control SC-derived myoblasts or SC-derived myoblasts transfected with mimic or inhibitors. Differences were considered statistically significant when p < 0.05 (marked with asterisks, *p < 0.05; **p < 0.005; ***p < 0.001).
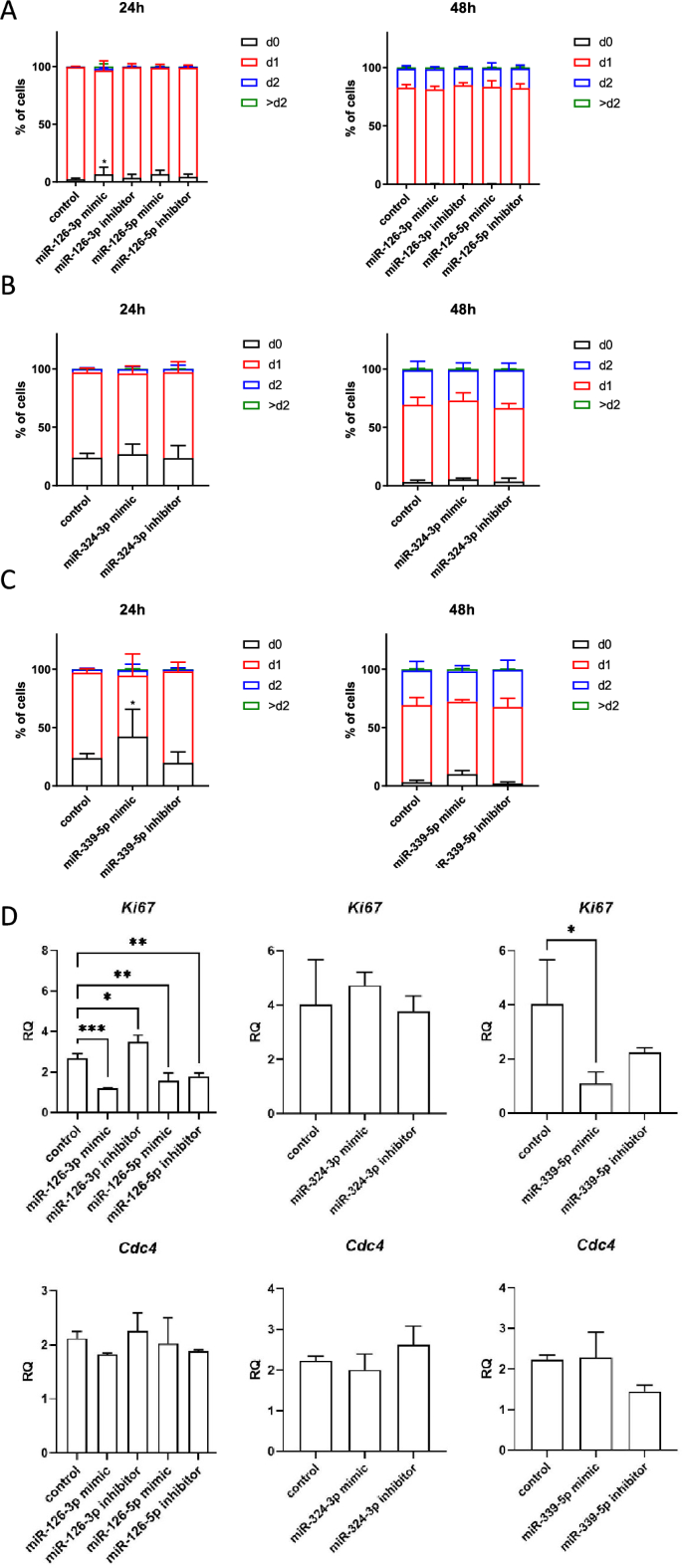
The influence of miRNA mimics and inhibitors transfection on SC-derived myoblasts proliferation, migration, and fusion
The proliferation of SC-derived myoblasts transfected with either miR-126-3p, or miR-126-5p, or miR-324-3p, or miR-339-5p mimics or microRNA inhibitors was analyzed using the flow cytometry of cells cultured in the presence of CFSE for 24 h or 48 h (Fig. 2A–C). No significant changes in proliferation of SC-derived myoblasts transfected with mimics or inhibitors, as compared to control, were observed at selected time points. The only exception were the cells transfected with miR126-3p or miR339-5p mimics. In this case we observed decreased proliferation at 24 h (Fig. 2A–C). In these cases, the number non-divided cells increased. Proliferation of SC-derived myoblasts was also assessed by the analysis of the expression of Ki67 and Cdc4 (encoding cell division control protein 4), i.e., the markers of dividing cells (Fig. 2D). The transfection of SC-derived myoblasts with either miR-126-3p, or miR-126-5p, or miR-339-5p mimics or miR-126a-5p inhibitor significantly decreased the Ki67 mRNA level. Furthermore, transfection with the miR-126a-3p inhibitor increased Ki67 mRNA level. However, the level of Cdc4 mRNA did not differ, as compared to control cells. Altogether, this analyses suggest that miR126-3p and miR-339-5p could impact SC-derived myoblast proliferation.
Impact of miR-126-3p, miR-126-5p, miR-324-3p, and miR-339-5p on mouse SC-derived myoblast proliferation. CFSE assay of control SC-derived myoblasts or SC-derived myoblasts transfected with miRNA mimics or inhibitors 24 h or 48 h after adding CFSE; (A) SC-derived control myoblasts and SC-derived myoblasts transfected with miR-126-3p or miR-126-5p mimics or their inhibitors; (B) SC-derived control myoblasts and SC-derived myoblasts transfected with mimic or inhibitor of miR-324-3p; (C) SC-derived control myoblasts and SC-derived myoblasts transfected with mimic or inhibitor of miR-339-5p. The proportion of cells that underwent one (d1), two (d2) or more (> d2) divisions were shown; (D) the level of Ki67 and Cdc4 transcripts in control SC-derived myoblasts and SC-derived myoblasts transfected with miR-126-3p, miR-126-5p, miR-324-3p, and miR-339-5p mimics or their inhibitors. Differences were considered statistically significant when p < 0.05 (marked with asterisks, *p < 0.05).
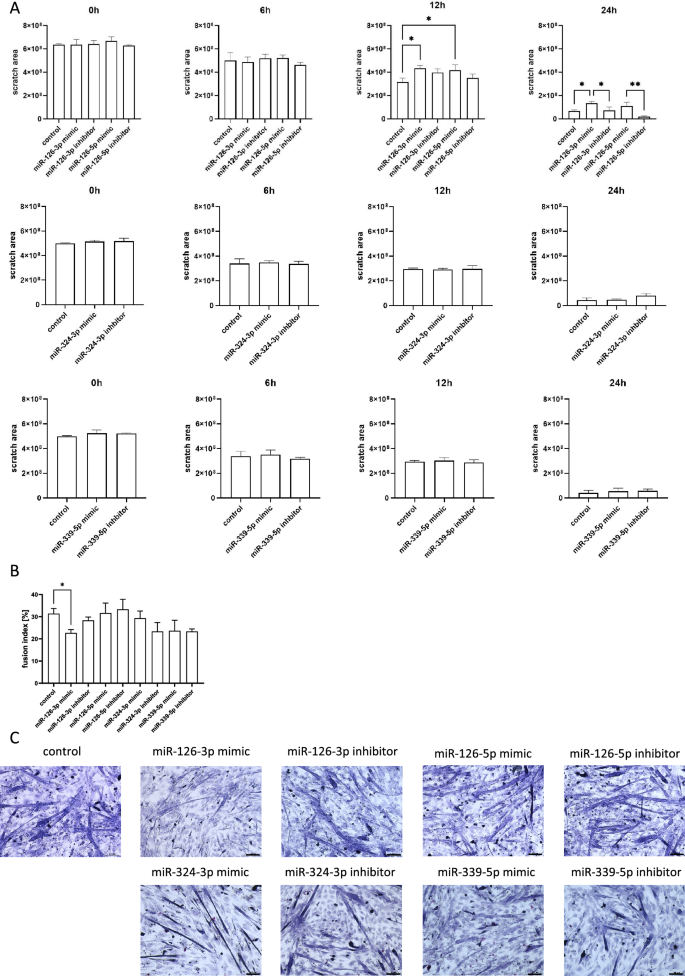
Then, the migration capacity of SC-derived myoblasts transfected with either miRNA126-3p, or miR-126-5p, or miR-324-3p, or miR-339-5p mimics or microRNA inhibitors was assessed using scratch assay (Fig. 3A). Migration was analyzed after 6, 12, and 24 h after the scratch was made. MiR-126-3p or miR-126-5p mimic transfected SC-derived myoblast migration was less effective than that of control cells (Fig. 3A). Furthermore, cells transfected with either miR-126-3p or miR-126-5p inhibitor migrated more effectively than those transfected with microRNA mimics. Changes in migration of SC-derived myoblast transfected with either miR-324-3p, or miR-339-5p mimics or inhibitors were not observed (Fig. 3A). To follow changes in SC-derived myoblast differentiation, the fusion index was analyzed (Fig. 3B,C). The SC-derived myoblasts were cultured in the presence of medium that supports myogenic differentiation for 5 days. The fusion index of SC-derived myoblast transfected with miR-126a-3p mimic was significantly lower than that of control cells (Fig. 3B,C). The fusion index of cells transfected either with miR-324-3p, or miR-339-5p mimics or their inhibitors did not differ, as compared to control ones (Fig. 3B,C).
Impact of miR-126-3p, miR-126-5p, miR-324-3p, and miR-339-5p on mouse SC-derived myoblast migration and fusion. (A) Migration of control or transfected SC-derived myoblasts analyzed 6, 12, and 24 h after performing a scratch; (B) Fusion index of control or transfected SC-derived myoblasts. Differences were considered statistically significant when p < 0.05 (marked with asterisks, *p < 0.05; **p < 0.005). (C) Representative images of control or transfected SC-derived myoblasts fusion.
The influence of miRNA mimics and inhibitors transfection on selected transcripts
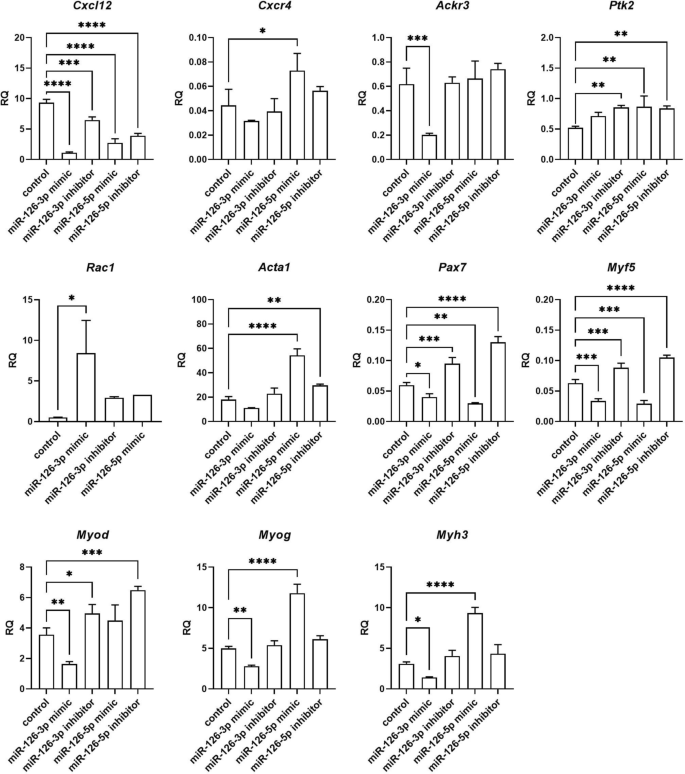
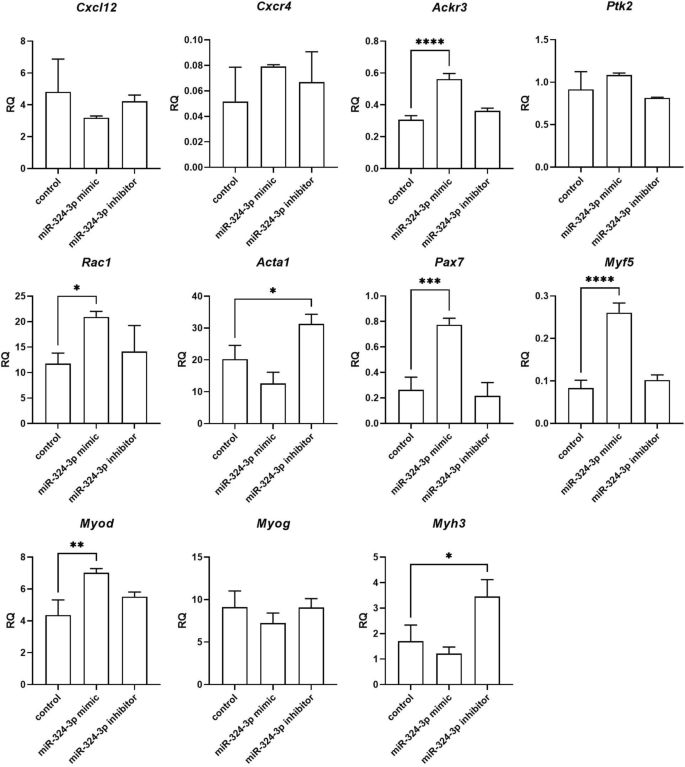
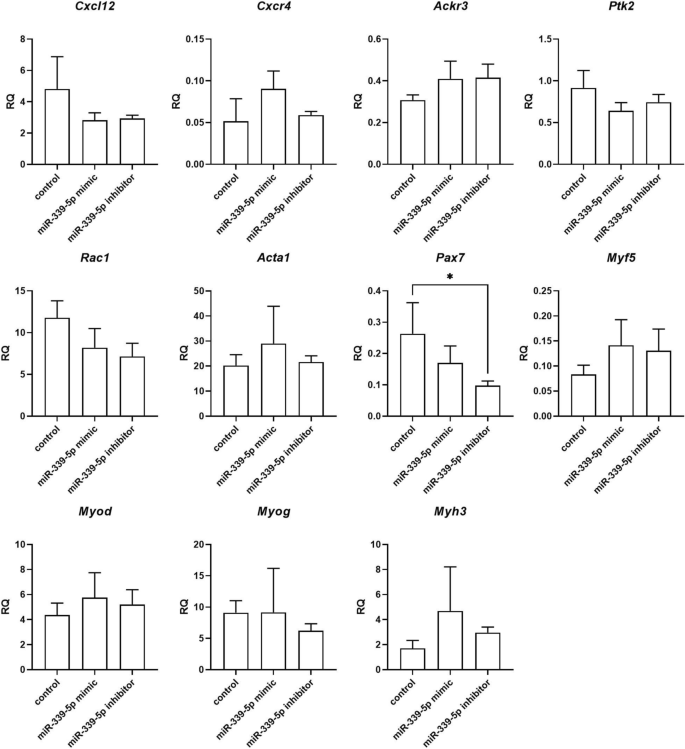
Changes in selected transcript levels were analyzed in SC-derived myoblasts transfected with either miRNA126-3p, or miR-126-5p, or miR-324-3p, or miR-339-5p mimics or inhibitors (Figs. 4, 5, 6, 7). The level of transcripts encoding proteins involved in SDF-1 signaling, cell proliferation, migration, and myogenic differentiation was examined. First, we focused on transcripts involved in SDF-1 signaling, such as Cxcl12, Cxcr4, and Ackr3 encoding SDF-1, CXCR4, and CXCR7, respectively. A significantly lower level of Cxcl12 and Ackr3 was observed in SC-derived myoblasts transfected with miR-126a-3p mimic (Fig. 4). A significantly lower level of Cxcl12 and the higher level of Cxcr4 was observed in SC-derived myoblast transfected with miR-126a-5p mimic (Fig. 4). Transfection with either miR-324-3p, or miR-339-5p mimics, or inhibitors did not change the level of transcripts encoding proteins involved in SDF-1 signaling, except for miR-324-3p mimic that increased the level of Ackr3 (Fig. 5). Changes in the level of transcripts encoding factors involved in cell proliferation were observed (Figs. 4, 5, 6). Then we analyzed the level of transcripts encoding factors involved in cytoskeleton reorganization and cell migration, such as Ptk2 and Rac1, which encode focal adhesion kinase (FAK) and Ras-related C3 botulinum toxin substrate 1 (RAC1), respectively (Figs. 4, 5, 6). Ptk2 expression increased significantly in SC-derived myoblasts transfected with either miR-126a-3p inhibitor, or miR-126a-5p mimic, or miR-126a-5p inhibitor. The level of Rac1 increased significantly in miR-126a-3p or miR-324-3p mimic transfected SC-derived myoblasts. Then, the levels of transcript encoding proteins engaged in myogenic differentiation, such as transcription factors: Pax7, Myod, Myf5, Myog, and structural protein transcripts of muscle cells, such as Acta1, Myh3 were analyzed (Figs. 4, 5, 6). Interestingly, miR-126a-3p mimic transfection significantly decreased the level of Pax7, Myod, Myf5, Myog, and Myh3 in SC-derived myoblasts. Furthermore, miR-126-5p mimic transfection significantly decreased the level of Pax7, Myf5 and increased the level of Myog, Myh3, and Acta1. The effect was reversed when miR-126a inhibitors were used. Furthermore, miR-324-3p mimic transfection significantly increased the level of Pax7, Myod, Myf5, and the miR-126-5p inhibitor increased the level of Acta1 and Myh3. In summary, miR-126-3p mimic transfection impacted the level of transcripts encoding factors involved in SDF-1 signaling, proliferation, migration, and myogenic differentiation and corresponds to changes observed during proliferation, migration, and fusion. The effect of miR-324-3p was observed mainly in case of myogenic factor transcript levels. However, these changes did not translate into differences in the fusion index. Thus, in further experiments we focused on the miR-126 function.
Relative expression of selected transcripts of control mouse SC-derived myoblasts or SC-derived myoblasts transfected with miR-126-3p or miR-126-5p mimics or their inhibitors. Differences were considered statistically significant when p < 0.05 (marked with asterisks, *p < 0.05; **p < 0.005; ***p < 0.001; ****p < 0.0001).
Relative expression of selected transcripts of control mouse SC-derived myoblasts and SC-derived myoblasts transfected with miR-324-3p mimic or its inhibitor. Differences were considered statistically significant when p < 0.05 (marked with asterisks, *p < 0.05; **p < 0.005; ***p < 0.001; ****p < 0.0001).
Relative expression of selected transcripts of control mouse SC-derived myoblasts and SC-derived myoblasts transfected with miR-339-5p mimic or inhibitor. Differences were considered statistically significant when p < 0.05 (marked with asterisks, *p < 0.05; **p < 0.005; ***p < 0.001; ****p < 0.0001).
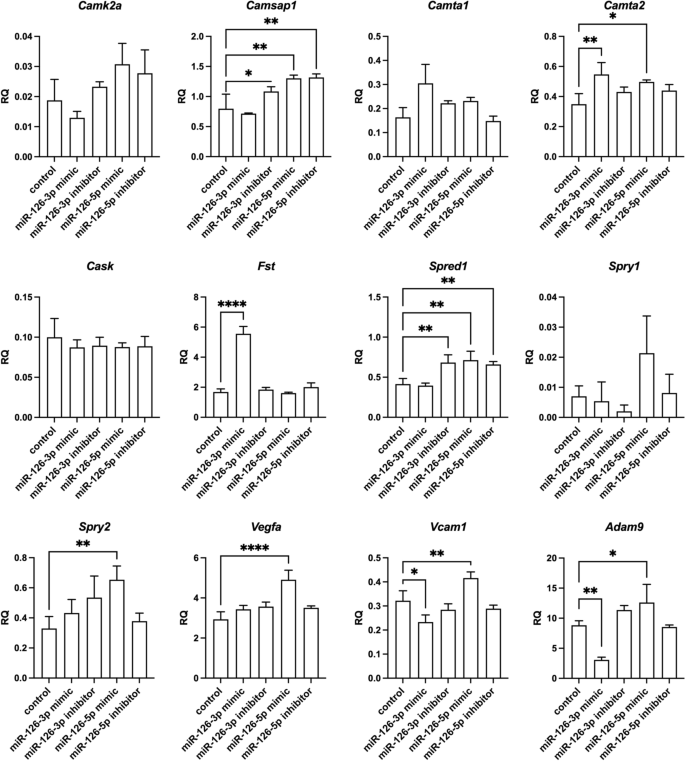
Relative expression of transcripts predicted by databases to be regulated by miR-126-3p or miR-126-5p of control mouse SC-derived myoblasts and SC-derived myoblasts transfected with miR-126-3p or miR-126-5p mimics or their inhibitors. Differences were considered statistically significant when p < 0.05 (marked with asterisks, *p < 0.05; **p < 0.005; ***p < 0.001; ****p < 0.0001).
The miR-126 targets
According to miR Data Base, Target Scan Mouse data base, mirWalk2, mirTarbase, among the predicted miR-126 targets are transcripts encoding adhesion proteins: Adam9 (a disintegrin and metallopeptidase domain 9), Vcam1; cytokine and growth factors: Vegfa, Cxcl12, Cxcr4, Fst (follistatin), Spred1 (sprouty protein with EVH-1 domain 1), Spry1 and Spry2 (Sprouty RTK signaling antagonist 1), calcium/calmodulin dependent proteins: Camk2a (calcium/calmodulin dependent protein kinase II alpha), Camsap1 (calmodulin regulated spectrin-associated protein 1), Camta1 and Camta2 (calmodulin-binding transcription activator), Cask serine protein kinase). As described above, we confirmed the down-regulation of Cxcl12 in miR-126-3p or miR-126-5p mimic transfected SC-derived myoblasts. Then we focused on other miR-126 targets and analyzed its level in transfected SC-derived myoblasts (Fig. 7). We did not observe down-regulation of Camk2a, Camsap1, Camta1, Camta2, Cask, Fst, Spred1, Spry1, or Spry2 . However, the level of Camsap1 and Spred1 increased in miR-126-3p inhibitor transfected SC-derived myoblasts. Furthermore, the level of Camta1, Camta2, Fst increased in miR-126-3p mimic transfected SC-derived myoblasts. However, the level of Adam9 and Vcam1 decreased significantly in miRNA-126a-3p mimic transfected SC-derived myoblasts. Importantly, Adam9, Vcam1, and Vegfa levels increased significantly in miRNA-126a-5p mimic transfected SC-derived myoblasts.
The impact of miR-126 transfected SC-derived myoblasts conditioned medium on endothelial cells tubulogenesis
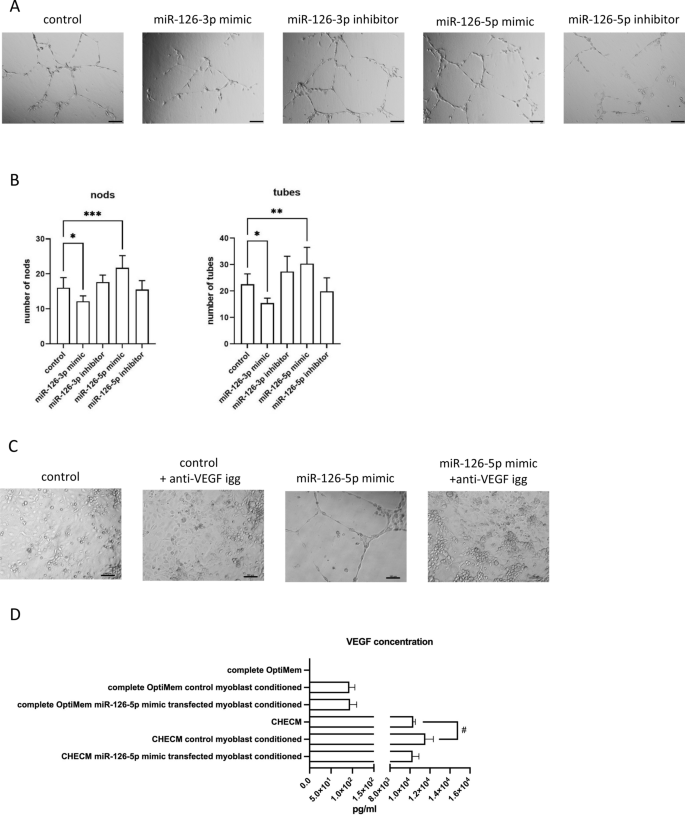
In the present study, we showed that myoblast transfection with miR-126-5p, which expression significantly increased after SDF-1 treatment, increased Vefga level (Fig. 7). To assess the functional impact of miR-126-5p treated SC-derived myoblasts on endothelial cells we used primary human skeletal muscle microvascular endothelial cells (PMECs). Importantly, treatment of PMECs with medium conditioned by miR-126-5p transfected SC-derived myoblasts promoted tubulogenesis (Fig. 8A,B). On the other hand, the presence of medium conditioned by miR-126-3p transfected SC-derived myoblast inhibited endothelial cell differentiation (Fig. 8A,B). Next, the PMECs were cultured in miR126-5p transfected myoblast conditioned medium in presence of anti-VEGF antibody (Fig. 8C). PMEC tubules were formed in miR126-5p transfected myoblast conditioned medium already after 16 h. At the same time control myoblast conditioned medium did not promote tubule formation. Moreover, PMEC tubulogenesis was impaired in the presence of anti-VEGF antibody, both in control myoblast and miR126-5p transfected myoblast conditioned medium. Next, the level of VEGF protein was analyzed in medium conditioned by control or miR126-5p transfected myoblast (Fig. 8D). The increased level of VEGF was detectable in medium conditioned by myoblasts comparing to complete medium (Fig. 8D). However, we were not able to detect difference in VEGF protein level between control myoblast conditioned medium and miR126-5p transfection did not impact VEGF secretion by myoblast. Therefore, we concluded that miR-126-5p, up-regulated in response to SDF-1 treatment, changed the secretome of myoblasts and what impacted endothelial cell function. However, apart of VEGF additional molecules could be engaged in this effect.
Impact of medium conditioned by the mouse control SC-derived myoblast or SC-derived myoblast transfected with miR-126-3p and miR-126-5p mimics or their inhibitors on PMECs. (A) Representative images of tube formation by PMECs cultured in control medium or medium collected from control or transfected SC-derived myoblasts (18 h); (B) Number of branching points (nods) and number of tubes formed by PMECs cultured in control medium or medium collected from control or transfected SC-derived myoblasts (18 h). Differences were considered statistically significant when p < 0.05 (marked with asterisks, *p < 0.05; **p < 0.005; ***p < 0.001). Scale: 100 µm. (C) The impact of anti-VEGF antibody present in culture medium or medium conditioned by the mouse control SC-derived myoblast or SC-derived myoblast transfected with miR-126-5p mimics on PMECs tubulogenesis (16 h). (D) The level of VEGF protein in complete OptiMem or Complete Human Endothelial Cell Medium (CHECM), or complete OptiMem collected from above control or transfected SC-derived myoblasts (48 h after transfection) or CHECM conditioned with control or transfected SC-derived myoblasts presence for 24 h. Differences were considered statistically significant when p < 0.05 (marked with asterisks, *p < 0.05; **p < 0.005; ***p < 0.001, when compared to OptiMem or marked with hashtag, #p < 0.05; ##p < 0.005; ###p < 0.001, when compared to CHECM).
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- ChartPrime. Elevate your Trading Game with ChartPrime. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://www.nature.com/articles/s41598-023-41626-z