Incisor morphology, strength, and enamel composition in Mast4 KO mice
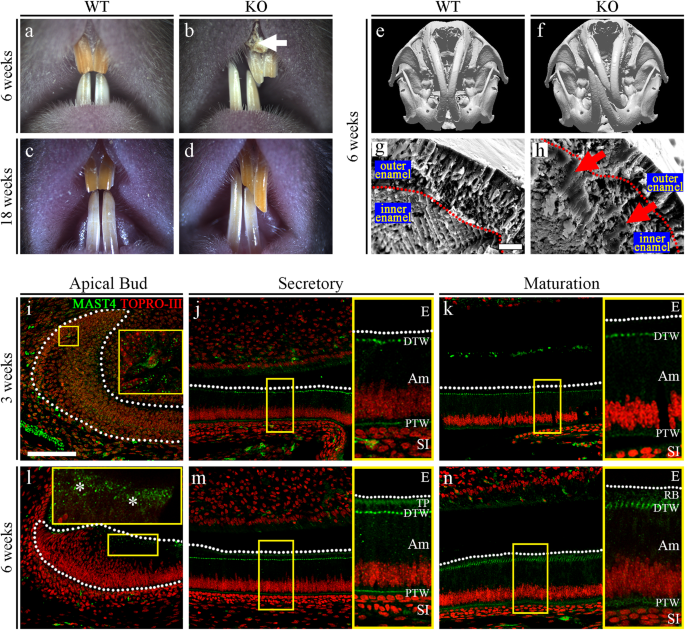
Mast4 KO mice with targeted deletion of 71 base pairs in exon 1, which resulted in a premature stop codon resulting from a frameshift mutation, were generated using the CRISPR/Cas9 system (Supplementary Fig. 1). Mast4 KO mice were grossly similar to their WT littermates after birth in terms of size and shape, particularly those of the cranial skeleton (Supplementary Fig. 2a). However, the maxillary and mandibular incisors of Mast4 KO mice showed asymmetrical attrition at postnatal week 3 (Supplementary Fig. 2b, arrow). At 6 weeks, the asymmetrical attrition in Mast4 KO mice became more severe, and bending began to occur (Fig. 1a, b). At 18 weeks, the Mast4 KO mice were all viable, but they possessed opaque mandibular incisors with chalky surfaces, while the mandibular incisors of WT mice were transparent and glossy (Fig. 1c, d).
a–d Comparison of incisors from 6-week-old and 18-week-old WT and Mast4 KO mice. Bent maxillary and mandibular incisors were observed in Mast4 KO mice. The arrow indicates the peeled enamel in a maxillary incisor from a 6-week-old Mast4 KO mouse. e, f Micro-CT 3D reconstruction of the head of a Mast4 KO mouse shows that the incisor is severely curved. g, h Scanning electron microscopy (SEM) images of incisor enamel dissected planes. The outer enamel of Mast4 KO incisors was thicker than that of WT incisors. Decussated enamel rods were observed in the inner enamel layer of the WT incisor. The enamel rod arrangement was collapsed in the Mast4 KO incisor (arrows). i–n MAST4 localization in the WT incisor. The dotted lines indicate the boundary of the epithelial cell layer. i–k In incisors from 3-week-old mice, MAST4 was generally weakly expressed at the apical bud, but single cells with high MAST4 expression in the stem cell niche region were also detected. MAST4 was weakly expressed at proximal terminal web complexes (PTW) and moderately distal terminal web complexes (DTW) in secretory and maturation stage ameloblasts. l After 6 weeks, MAST4 expression extended to the apical side (asterisks). m MAST4 was localized to both sides (proximal and distal) of terminal web complexes and Tomes’ processes (TP) in secretory stage ameloblasts. n In the ameloblast maturation stage, MAST4 was observed at the ruffled border (RB) and terminal web complexes (PTW, DTW). E enamel, DTW distal terminal web complex, PTW proximal terminal web complex, TP Tomes’ process, RB ruffled border, Am ameloblast, SI stratum intermedium. Scale bars, g, h, 10 μm; l–n, 100 μm.
We also observed twisting to one side between the maxillary and mandibular incisors in 80 to 90% of the Mast4 KO mice; the incisors were overgrown, and the enamel of the maxillary incisors was peeled off (Fig. 1b, arrow). Microcomputed tomography (μCT) analysis of the head more clearly revealed the incisor malocclusion in Mast4 KO mice (Fig. 1e, f). To investigate the developmental patterns of molar dentition, histological analysis was performed at the bell stage (embryonic day 18.5; Supplementary Fig. 3a, b, d, e). Relative to that in the molar tooth germs of WT mice, the ameloblast layer arrangement in the molar tooth germs of Mast4 KO mice exhibited an irregular shape (Supplementary Fig. 3e, arrowheads). However, there was no difference in morphogenesis, including in cuspal patterning. In addition, there were no differences in molar dentition between WT and Mast4 KO mice until postnatal week 10 (Supplementary Fig. 3c, f).
Scanning electron microscopy (SEM) analysis was performed to compare the developing enamel between WT and Mast4 KO mice (Fig. 1g, h and Supplementary Fig. 4). This revealed that the enamel of developing Mast4 KO mouse incisors contained a collapsed enamel rod arrangement in the inner enamel (Fig. 1h, arrows), specifically in the area adjacent to the outer enamel (Supplementary Fig. 4, arrowheads). Furthermore, electron probe microanalysis (EPMA) was performed to investigate the mineral content of the incisors (Supplementary Fig. 5a, b). We divided the calcified portion of incisors from 6-week-old mice into two parts (the secretory and maturation regions) and proceeded with EPMA. Interestingly, in the maturation region of Mast4 KO incisors, calcium (Ca), magnesium (Mg), and phosphorous (P) were reduced, whereas there were no significant differences in the secretory region. We then performed a Vickers test to determine the hardness of the incisors of 6-week-old WT and Mast4 KO mice. The Vickers test revealed that the loss of MAST4 expression significantly reduced enamel hardness (Supplementary Fig. 6). This altered intensity cannot balance the incisor bite force and may lead to a twisted phenotype. Based on these results, the expression pattern of MAST4 was confirmed in various developmental stages of molars and incisors (Supplementary Fig. 7) as well as in three different regions of juvenile (3-week-old) and adult (6-week-old) incisors (Fig. 1i–n). At the E12.5, E14.5, and E16.5 stages, MAST4 was not detected in molars (Supplementary Fig. 7a–c). At postnatal 1 day, while MAST4 was expressed and dispersed in the stellate reticulum (SR), its expression was not detected in ameloblasts (Supplementary Fig. 7d). In the incisors, there was no MAST4 expression at any developmental stage (Supplementary Fig. 7e, f). At 3 weeks, MAST4 was generally weakly expressed, but strong MAST4 expression was observed in a single cell in the stem cell niche of the apical bud (Fig. 1i). However, MAST4 was expressed at proximal and distal terminal web complexes in the secretory and maturation stages (Fig. 1j, k, PTW, DTW). At 6 weeks, MAST4 expression was observed on the apical side (Fig. 1l, asterisks). In particular, MAST4 was detected at Tomes’ processes (Fig. 1m, TP) as well as
terminal web complexes (Fig. 1m, PTW, DTW) in the secretory stage and at the ruffled border of ameloblasts (Fig. 1n, RB) and terminal web complexes (Fig. 1n, PTW, DTW) in the maturation stage. These results suggest that the weakness of the incisors of Mast4 KO mice compared to those of WT mice may be caused by dysregulation during amelogenesis, not during development.
Amelogenesis dysregulation in the incisors of Mast4 KO mice
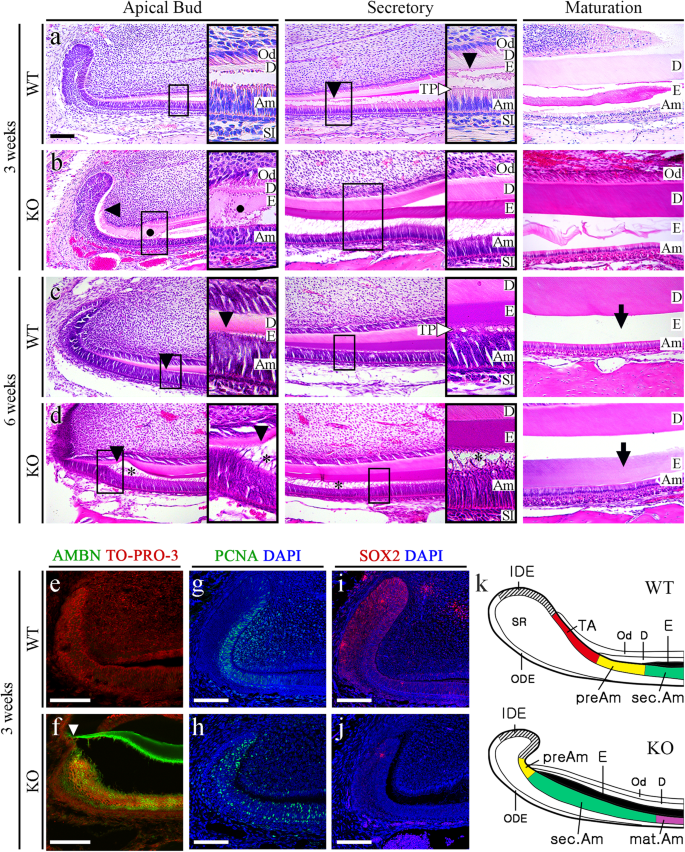
The apical buds of mouse incisors contain an epithelial stem cell niche that provides the inner dental epithelium (IDE) cells that differentiate into ameloblasts. To investigate stem cell differentiation, apical bud regions were dissected from the mandibles of 6-week-old WT and Mast4 KO mice (Supplementary Fig. 8a, b). The initiation of enamel matrix secretion was shifted to the apical side of the Mast4 KO incisors (Supplementary Fig. 8b). Interestingly, the apical bud of the Mast4 KO incisor appeared to be reduced (Supplementary Fig. 8b, dotted line). In particular, the TA zone seemed to have disappeared. We investigated the histology of the incisor teeth in decalcified sagittal sections from WT and Mast4 KO mice at 3 and 6 weeks of age (Fig. 2a–d). In WT mice, secretory stage ameloblasts were tall and columnar, and enamel matrix proteins were present in the forming enamel (Fig. 2a). During the maturation stage, WT mice exhibited characteristic shortened ameloblasts. In contrast, epithelial cells in the TA zone of the apical bud region appeared to have been transformed into secretory ameloblasts in the KO incisors (Fig. 2b). The initiation of enamel matrix secretion was shifted to the apical bud region in the Mast4 KO incisors, while the initiation of secretion started in secretory stage ameloblasts in the WT incisors (Fig. 2a, b, arrowheads). In particular, ectopic atypical enamel matrix deposition without underlying dentin formation was detected in 40 to 50% of Mast4 KO mice (Fig. 2b, circle). In the maturation stage, severe hypomineralization was detected in the Mast4 KO incisor enamel (Fig. 2b Maturation). The initiation position of dentin matrix secretion did not differ between the WT and KO incisors. At 6 weeks, the initiation of enamel deposition in the KO incisors was shifted to the apical as observed at 3 weeks (Fig. 2c, d, arrowheads). Additionally, compared with the normal Tomes’ processes in WT mice (Fig. 2c, TP), the Tomes’ processes in Mast4 KO mice were stretched through the apical bud region to the secretory region at 6 weeks (Fig. 2d, asterisks). The degradation and absorption of enamel matrix proteins, important functions of maturation stage ameloblasts, were performed properly, and the enamel space appeared empty in the WT incisors (Fig. 2c, arrow). However, the eosinophilic staining of the enamel space in Mast4 KO incisors indicated that these functions were not performed properly in KO incisor ameloblasts and that significant amounts of enamel proteins remained (Fig. 2d, arrow). Enamel matrix proteins are substituted during enamel maturation, and the proportion of these proteins in mature enamel is known to be less than 2% m/m42. This feature of excessive remaining enamel proteins was consistent with the low mineral composition (high protein composition) and peeled enamel observed in Mast4 KO mice (Fig. 1b, arrow and Supplementary Fig. 4). Ectopic enamel secretion was verified by evaluating ameloblastin expression (Fig. 2e, f), and ameloblastin was found to be expressed and released to the enamel layer on the apical side in Mast4 KO incisors (Fig. 2f, arrowhead), suggesting that enamel matrix secretion is accelerated temporarily which resulted in advanced spatially in Mast4 KO incisors. Proliferating Cell Nuclear Antigen (PCNA), a TA cell marker in incisors, was expressed in the TA region of the epithelium and adjacent mesenchyme of the WT incisors, whereas PCNA was scattered throughout the epithelium and sparsely detected in the mesenchyme of the KO incisors (Fig. 2g, h). SOX2, which is a marker of dental epithelial stem cells (DESCs), was expressed throughout the WT apical bud, including in TA cells (Fig. 2i). However, SOX2 was detected in a limited area known as the stem cell niche in the apical bud of the KO incisors (Fig. 2i, j). Schematic diagrams showing these features of WT and Mast4 KO incisors and a summary of the histological observations are presented (Fig. 2k).
a, b HE-stained images of incisors from 3-week-old WT and Mast4 KO mice. The black arrowheads indicate the position of enamel deposition initiation. In terms of the amelogenesis stages, early enamel deposition was observed in the Mast4 KO incisor compared to the WT incisor. a In the secretory and maturation stages, enamel and ameloblasts fully contacted each other via the Tomes’ process (white arrowhead). b In contrast to the WT incisor, the ectopic atypical enamel deposition in the Mast4 KO incisor began at the apical region (black circle). In the maturation stage, hypomineralization was detected in the Mast4 KO incisor. c, d HE-stained images of incisors from 6-week-old WT and Mast4 KO mice. Early enamel deposition was also observed (black arrowheads). In the secretory stage, the Tomes’ processes of ameloblasts fully contacted the enamel in WT mice (white arrowhead), whereas the Tomes’ processes were stretched and destroyed through the apical bud region to the secretory stage region in KO mice (asterisks). After decalcification, c the enamel in the WT maturation stage contained a lower abundance of enamel matrix proteins than d the enamel in the Mast4 KO maturation stage (arrows). (d maturation) The separation of enamel and dentin was an artifact that occurred during sample sectioning. e, f Ameloblastin (AMBN) localization in incisors from 3-week-old WT and Mast4 KO mice. f AMBN was expressed and released to the enamel layer on the apical side in the Mast4 KO incisor compared to the WT incisor. The white arrowhead indicates the position of enamel deposition initiation. g, h The localization of the TA cell marker PCNA in the apical bud region of WT and Mast4 KO incisors. PCNA was localized densely in the TA region and adjacent mesenchyme in the WT but showed a scattered distribution in the Mast4 KO apical bud. i, j The DESC marker SOX2 was expressed throughout the apical bud of the WT incisor and in a limited small area of the apical bud of the Mast4 KO incisor. k Schematic diagrams of the mandibular labial incisor epithelium in WT and Mast4 KO mice. In the mandibular incisors of Mast4 KO mice, the size of the labial apical bud, including the SR and IDE, was reduced. The TA region disappeared, and the initiation of enamel deposition was shifted to the reduced apical bud in Mast4 KO incisors. D dentin, E enamel, TP Tomes’ process, Am ameloblast, Od odontoblast, sec.Am secretory ameloblast, mat.Am maturative ameloblast, preAm preameloblast, IDE inner dental epithelium, ODE outer dental epithelium, SR stellate reticulum, TA transit-amplifying zone. All scale bars, 100 μm.
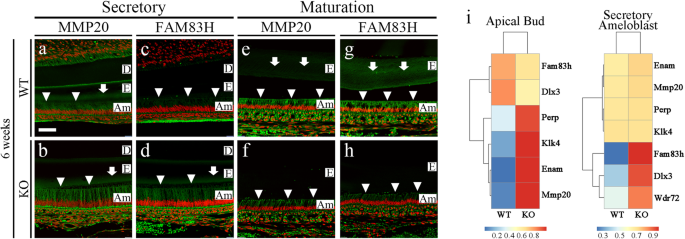
To determine and identify additional molecules involved in the disrupted amelogenesis in Mast4 KO mice, we screened MMP20 and FAM83H by immunofluorescence staining in 6-week incisors from WT and Mast4 KO mice (Fig. 3a–h). Interestingly, the expression of MMP20, which aids in enamel protein alignment in the enamel matrix and ameloblasts, did not differ between WT and Mast4 KO mice (Fig. 3a, b). However, FAM83H secretion into the enamel matrix was significantly increased in the Mast4 KO secretory region (Fig. 3c, d). In the maturation region, the quantity of both proteins in the enamel matrix was lower in Mast4 KO mice than in WT mice (Fig. 3e-h). In particular, the expression of FAM83H, which plays a key role in enamel maturation, shifted to an early stage in the secretory region (Fig. 3d, g, arrows and arrowheads). These results are consistent with the shifted initiation of enamel secretion and reduced size of the apical bud region in the Mast4 KO incisors.
a–h Enamel matrix protein (MMP20) and maturation-related protein (FAM83H) in the secretory and maturation regions of incisors from 6-week-old WT and Mast4 KO mice. The arrowheads indicate ameloblasts expressing MMP20 and FAM83H. The arrows indicate the expression of enamel matrix proteins. a, b In the secretory region, the expression of MMP20 was similar between the WT and Mast4 KO mice. c, d FAM83H expression was increased in Mast4 KO ameloblasts. e–h In the maturation region, MMP20 and FAM83H expression was decreased in ameloblasts as well as in the enamel matrix in Mast4 KO mice. i RNA-Seq analyses of apical buds and secretory ameloblasts from the incisors of 6-week-old WT and Mast4 KO mice. At the apical bud, the expression of Perp, Klk4, Enam (Enamelin), and Mmp20 was increased in Mast4 KO incisors. In the ameloblast region, the expression of Fam83h, Dlx3, and Wdr72 was increased in Mast4 KO incisors. D dentin, E enamel, Am ameloblast. Scale bar; 50 μm.
We further performed RNA sequencing analysis of two regions apical buds and secretory ameloblasts, to compare 6-week-old WT incisors and Mast4 KO incisors (Fig. 3i). The major challenge can be considered to be the definition of the Mast4 KO apical bud region (properties of secretory ameloblasts and apical buds) and the secretory ameloblast region (properties of maturative ameloblasts) due to the reduction in the apical bud size in Mast4 KO mice. Consequently, transcriptome analysis of secretory ameloblasts (Fig. 3i) represented an area of an exact match, as shown in Fig. 3a–d. In the apical bud region, the expression of enamel matrix protein-encoding genes, including Klk4, Enam, and Mmp20, was increased in Mast4 KO mice. In the secretory ameloblast region, the expression of enamel matrix maturation-related genes, including Fam83h, Dlx3, and Wdr72, was increased in Mast4 KO mice. These results suggest that enamel secretion and maturation end at an early stage of amelogenesis in the Mast4 KO incisor compared to the WT incisor.
Effects of Mast4 deficiency on the Wnt signaling pathway
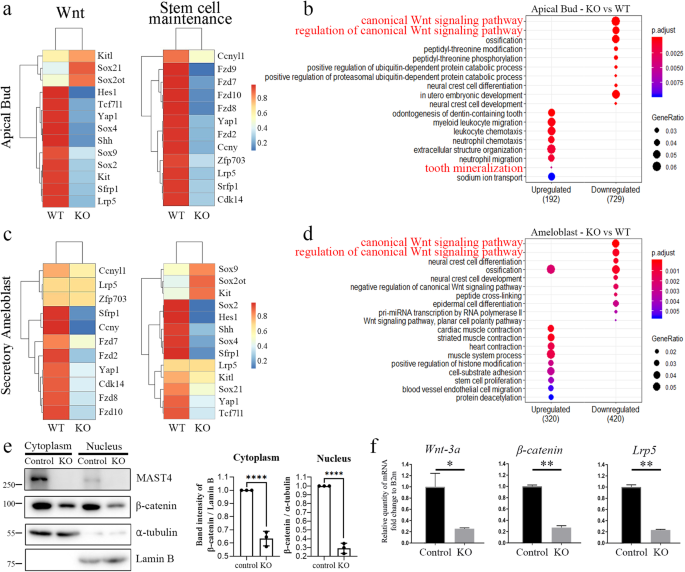
Via RNA sequencing analysis of secretory ameloblasts and apical buds isolated from 6-week-old WT and Mast4 KO incisors, changes in the Wnt signaling pathway profile and stem cell maintenance were detected. Changes in gene expression identified through analysis of differentially expressed genes (DEGs) were visualized on heatmaps, and large decreases in the expression of canonical Wnt signaling genes and stem cell maintenance-related genes were observed in both the apical bud and secretory ameloblast regions in Mast4 KO incisors (Fig. 4a, c). Gene Ontology (GO) analysis was performed on the RNA sequencing data (Fig. 4b, d). Canonical Wnt signaling was significantly downregulated in both the apical buds and ameloblasts of Mast4 KO mice. Interestingly, ossification was downregulated in Mast4 KO ameloblasts. Some tooth mineralization markers were upregulated in apical buds (Fig. 4b). We confirmed these changes in the Wnt signaling pathway in Mast4-null mHat9d cells, a dental epithelial stem cell line derived from the apical bud epithelium of a mouse incisor, finding that β-catenin expression was decreased in both the nucleus and cytoplasm (Fig. 4e). Mast4 ablation markedly decreased the transcription of Wnt signaling molecules, including Wnt-3a, β-catenin, and Lrp5 (Fig. 4f). This result suggests that ameloblast differentiation is accelerated due to altered Wnt signaling and stem cell maintenance in the apical bud region.
a–d RNA-Seq analyses of apical buds and ameloblasts from both WT and Mast4 KO incisors. a, c Heatmaps showing that canonical Wnt-related genes showed downregulation in Mast4 KO apical buds and ameloblasts. The expression of genes related to stem cell maintenance was also downregulated in Mast4 KO incisors. b, d GO analysis of both apical buds and ameloblasts revealed that the canonical Wnt signaling pathway was downregulated in Mast4 KO incisors. e Subcellular fractionation of control mHat9d cells and mHat9d cells with lentivirus-mediated Mast4 KO was performed. Notably, β-catenin expression in both the cytoplasm and nucleus was decreased in Mast4 KO cells (mean ± SD, n = 3). f Real-time PCR analysis of the expression of Mast4, Wnt-3a, β-catenin, and Lrp5 in mHat9d cells (mean ± SD, n = 3). The expression of Wnt-related genes was reduced after Mast4 KO in mHat9d cells. *p < 0.05, **p < 0.01, ****p < 0.0001.
MAST4 promoted the nuclear translocation of DLX3 by mediating serine/threonine phosphorylation of DLX3 within the nuclear localization signal (NLS)
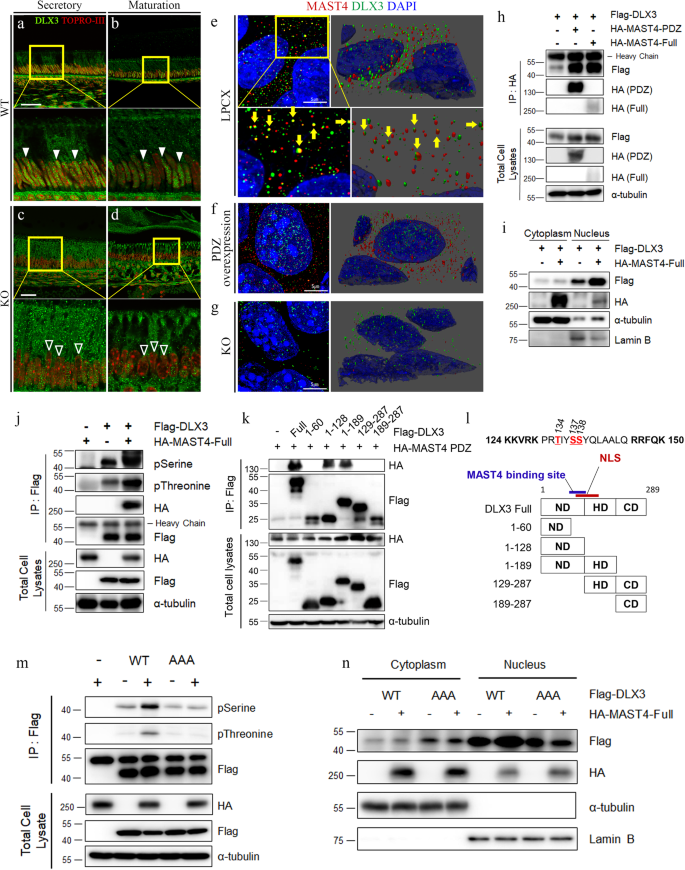
Considering that the phenotypes of enamel disruption and the significant reduction in the mineral content found in Mast4 KO mice were similar to the phenotypes observed in conditional Dlx3 KO mice30, we focused on examining the relationship between MAST4 and the DLX3 transcription factor, which plays an essential role in amelogenesis. First, we checked the distribution pattern of DLX3 in WT and Mast4 KO mice. The expression of DLX3 showed distinct localization patterns depending on the differentiation stage in the incisors of 6-week-old WT mice. DLX3 was predominantly located in the nuclei of ameloblasts at the secretory stage in the incisors of WT mice (Fig. 5a, arrowheads). At the maturation stage, DLX3 expression remained high in the nuclei of ameloblasts (Fig. 5b, arrowheads). However, in the incisors of Mast4 KO mice, DLX3 localization to the nucleus was decreased at both the secretory and maturation stages (Fig. 5c, d, blank arrowheads). These results suggest that the nuclear translocation of DLX3 might be impaired in Mast4 KO mice.
a–d DLX3 localization in incisors from 6-week-old WT and Mast4 KO mice. a, b In the WT incisor, DLX3 localized to the nucleus (arrowheads) and cytoplasm. c, d In the Mast4 KO incisor, the abundance of DLX3 in the nucleus was lower than that in the WT incisor (blank arrows). e–g MAST4 and DLX3 localization in empty LPCX vector-transfected control (e), MAST4 PDZ-overexpressing (f), and MAST4 KO (g) mHAT9d cells. Immunocytochemistry was performed, and confocal z-stack images were acquired in ultra-high-resolution mode (Lightning mode in Leica operating software). 3D reconstructions of the images were generated. The yellow arrows indicate direct binding of MAST4 and DLX3 in (e). h Immunoprecipitation was conducted after transient transfection of both HA-MAST4 PDZ and HA-MAST4-Full into HEK293T cells. i Analysis of DLX3 expression using subcellular fractionation following transient transfection of full-length MAST4 in the HEK293T cell line. The expression of α-tubulin in the cytoplasm and Lamin B in the nucleus served as controls for the efficiency of subcellular fractionation. j Flag-DLX3 was immunoprecipitated, and the complexes were analyzed by western blotting. Note that DLX3 phosphorylation was increased in the presence of HA-MAST4-Full in the HEK293T cell line. k HA-MAST4 PDZ and various Dlx3 deletion mutants were cotransfected into HEK293T cells, followed by immunoprecipitation. Note that MAST4 bound to the C-terminus of the ND. l Schematic diagram showing the deletion of the entire Dlx3 sequence. Note that the NLS is located at aa 124–150. m HEK293T cells were transiently cotransfected with Flag-DLX3WT, the DLX3AAA mutant and HA-MAST4-Full. Flag-DLX3 was immunoprecipitated, and the complexes were analyzed by western blotting. Note that DLX3 phosphorylation was increased in the presence of MAST4. n Flag-DLX3WT, the DLX3AAA mutant and HA-MAST4-Full were transiently cotransfected into HEK293T cells, and subcellular fractionation was performed. Notably, compared with that of DLX3WT, the proportion of the DLX3AAA mutant in the cytoplasm was higher and was not regulated by MAST4 (mean ± SD, n = 3). NLS nuclear localization site, ND N-terminal domain, HD homeodomain, CD C-terminal domain. Scale bars; a–d, 40 μm; e–g, 5 μm. The data were representative of three independent experiments.
Based on this observation, we examined whether MAST4 regulates the nuclear translocation of DLX3. Because of the high molecular weight (>285 kDa) of full-length MAST4 and its relatively low expression in mHat9d cells, we used a truncated Mast4 construct (MAST4 PDZ) containing the DUF, kinase, and PDZ domains that was shown to function normally in two previous studies37,38. To explore the relationship between MAST4 and DLX3, immunocytochemistry was performed for MAST4 and DLX3 in control, MAST4 PDZ-overexpressing, and MAST4-depleted mHAT9d cell lines. In the control (empty LPCX vector-transfected) cells, several MAST4 and DLX3 proteins directly bound to each other in the cytoplasm (Fig. 5e, yellow arrows and Supplementary Fig. 9a, d). Some DLX3 was detected in the nucleus, while most MAST4 was located in the cytoplasm. In MAST4 PDZ-overexpressing mHAT9d cells, DLX3 was translocated into the nucleus, and the abundance of MAST4 was increased in the cytoplasm (Fig. 5f and Supplementary Fig. 9b, e). A direct interaction between MAST4 and DLX3 was observed in these cells, but the number of complexes was decreased compared to that in the control cells. In MAST4-depleted cells, the abundances of MAST4 and DLX3 in the cytoplasm were not decreased (Fig. 5g and Supplementary Fig. 9c, f). The cross-sectional z-stack images acquired from these three cell types (LPCX, PDZ over, and KO), along with the related quantitative analysis, demonstrated a positive correlation between the MAST4 level and the degree of DLX3 nuclear translocation (Supplementary Fig. 9a–c, g). Additionally, an immunoprecipitation assay was performed in HEK293T cells, and interaction between MAST4 (both MAST4 PDZ and MAST4-Full) and DLX3 was detected (Fig. 5h). We next confirmed that DLX3 nuclear translocation increased when MAST4 was stably overexpressed in mHat9d cells (Supplementary Fig. 10a) or transiently overexpressed in HEK293T cells (Fig. 5i and Supplementary Fig. 10b). Considering that MAST4 functions as a serine/threonine kinase and that DLX3 is targeted by MAST4 for phosphorylation to regulate its DNA binding activity32, we examined whether MAST4 induces DLX3 phosphorylation. Interestingly, immunoprecipitation assays revealed that MAST4 overexpression significantly increased both the serine and threonine phosphorylation of DLX3 in both mHat9d (Supplementary Fig. 10e) and HEK293T cells (Fig. 5j and Supplementary Fig. 10c, d, f).
Next, to determine the sites of MAST4-mediated phosphorylation, multiple Dlx3 deletion mutants were generated, and we found that MAST4 bound to the C-terminus of the ND of DLX3, which is adjacent to the NLS region (aa 124–150) (Fig. 5k, l). In a previous report, it was revealed that the NLS region of DLX3 is a critical region for its nuclear translocation43. In addition, the mechanism by which phosphorylation adjacent to the NLS region regulates protein translocation has been elucidated by performing serine-to-alanine mutations of the target residues44,45. Therefore, to investigate whether MAST4-mediated phosphorylation adjacent to the NLS of DLX3 is necessary for its nuclear translocation, we generated a nonphosphorylatable mutant with alanine substitutions of the serine and threonine residues (T134, S137, and S138) within the NLS region (DLX3AAA, Supplementary Fig. 16a). Interestingly, while MAST4 overexpression significantly increased serine and threonine phosphorylation of DLX3WT, the phosphorylation of the DLX3AAA mutant was not affected by MAST4 overexpression in HEK293T cells (Fig. 5m and Supplementary Fig. 11). More DLX3AAA than DLX3WT was localized to the cytoplasm, but the nuclear translocation of DLX3AAA was not promoted by MAST4 (Fig. 5n and Supplementary Fig. 12). Furthermore, we confirmed that the expression of carbonic anhydrase genes, such as Car6 and Car12, and ion transporter genes, such as Slc26a1 and Slc34a2, in Mast4 KO mHat9d cells was rescued through the overexpression of DLX3 (Supplementary Fig. 13). These results suggest not only that phosphorylation of the DLX3 NLS by MAST4 is important for the nuclear translocation of DLX3 but also that the abnormal enamel secretion and maturation phenotypes observed in Mast4 KO mice are mediated through the regulation of DLX3 by MAST4.
Considering the previously reported correlation between DLX3 and Wnt signaling, we investigated whether the alterations in Wnt signaling observed in Mast4 KO mice are associated with the abnormal translocation of DLX3. Interestingly, in mHat9d cells, transient overexpression of DLX3 did not differentially regulate Wnt signaling, and DLX3 did not exhibit any specific effect on the inhibition of Wnt signaling caused by Mast4 KO (Supplementary Fig. 14). Therefore, further research is needed to elucidate the relationship between DLX3 and Wnt signaling. Additionally, we confirmed that the changes in Wnt signaling and DLX3 activity observed in Mast4 KO mice were independent of each other.
Phosphorylation of the DLX3 NLS by MAST4 regulated the activation of DLX3 target genes involved in pH regulation
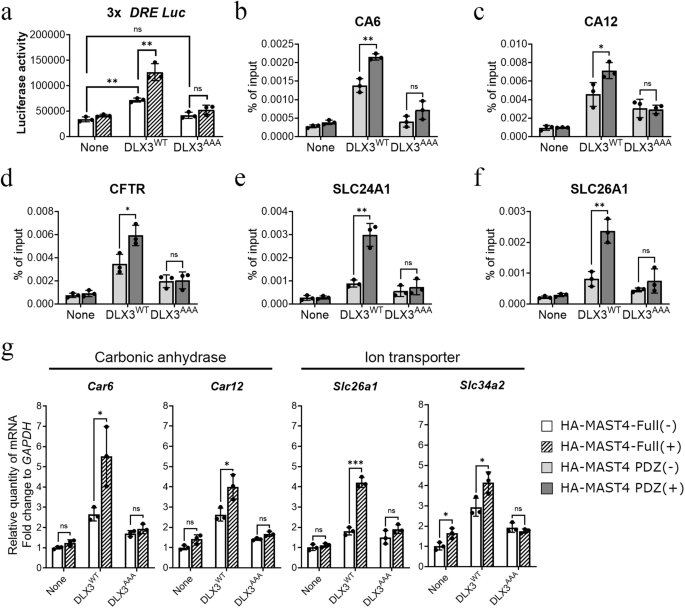
To understand the functional implications of phosphorylation of the NLS of the DLX3 transcription factor, a luciferase reporter assay using pGL3-3xDRE, which contains three copies of the DLX3-responsive elements, was performed to assess the transcriptional activity of both DLX3WT and DLX3 mutants in HEK293T cells46. As expected, the basal transcriptional activity of DLX3WT was greater than that of the DLX3AAA mutant (Fig. 6a and Supplementary Fig. 15). In particular, the transcriptional activity of DLX3WT was further increased when MAST4 was overexpressed, whereas the effect of MAST4 overexpression on the transcriptional activity of the DLX3AAA mutant was relatively not significant. In addition, the phosphomimetic DLX3EEE mutant with glutamic acid substitutions further increased the basal transcriptional activity of DLX3 without being affected by MAST4 overexpression, indicating that the phosphorylation status of the NLS of DLX3 is critical for activation of its target genes (Supplementary Fig. 16b). Next, we investigated whether the occupancy of each target gene promoter by DLX3 is regulated by MAST4-mediated NLS phosphorylation. In a previous report, direct target genes of DLX3, such as carbonic anhydrase and ion transporter genes involved in pH regulation, were identified, and DLX3 binding sites in the promoter of each target gene were also identified30. With reference to a previous report, chromatin immunoprecipitation (ChIP) assays were conducted to examine the binding of DLX3 to its target gene promoters in both HEK293T and mHAT9d cells. Interestingly, in the case of carbonic anhydrase genes (CA6 and CA12) and ion transporter genes (CFTR, SLC24A1, and SLC26A1), DLX3WT exhibited increased occupancy compared with that of DLX3AAA (Fig. 6b–f and Supplementary Fig. 17). In particular, the occupancy of DLX3WT on the target gene promoters was further increased by MAST4 PDZ overexpression, while that of DLX3AAA was not affected. Next, to confirm whether the mRNA levels of the target genes are regulated by altered translocation of DLX3 via MAST4, RT‒qPCR was performed in mHat9d cells. The activation of the carbonic anhydrase target genes Car6 and Car12 and the ion transporter target genes Slc26a1 and Slc34a2 was significantly downregulated by the expression of DLX3AAA, while expression of the DLX3EEE mutant further increased target gene activation (Fig. 6g and Supplementary Fig. 16c). Consistently, a MAST4 PDZ-mediated increase in target gene activation was observed when DLX3WT was transiently co-overexpressed, while neither of the two DLX3 mutants was further differentially regulated, confirming the NLS phosphorylation site-specific role of Mast4. These results indicate that phosphorylation of the DLX3 NLS by MAST4 plays an important role in promoting the nuclear translocation of DLX3 and subsequent activation of the target genes.
a 3xDRE-luc, DLX3, and HA-MAST4-Full were transiently overexpressed in HEK293T cells, and beta-galactosidase was cotransfected for normalization. Luciferase activities were measured after 48 h. b–f DLX3WT, DLX3AAA, and HA-MAST4 PDZ were transiently transfected into HEK293T cells. ChIP assays showed that transfection of DLX3WT increased target gene promoter binding and that HA-MAST4 PDZ cotransfection further increased target gene promoter binding, whereas transfection of DLX3AAA had no significant effect. b–d Carbonic anhydrases. e, f Ion transporters. g RT‒qPCR results for carbonic anhydrases and ion transporters involved in pH regulation. mHat9d cells were transiently transfected with DLX3WT, DLX3AAA, and HA-MAST4-Full. The data were presented as the means ± SDs (n = 3 for a–g). *p < 0.05; **p < 0.01; ns nonsignificant.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s12276-024-01264-5