<!–
–>
Nonalcoholic fatty liver disease (NAFLD) is a condition in which there is a build-up of excess fat in the liver cells. When left untreated, NAFLD can cause inflammation and damage to the liver, leading to a more serious condition known as nonalcoholic steatohepatitis (NASH). Although the early identification of NAFLD/NASH is essential for the reversal of disease progression, the lack of symptoms experienced during the initial stages of NAFLD/NASH makes it difficult to diagnose patients for years or even decades. Therefore, NASH is not usually diagnosed until the liver becomes fibrotic or scarred. As it is well known, the NASH market remains lucrative due to the disease’s increasing prevalence and lack of approved therapies in the US and EU. While it is possible that this may change soon, with Madrigal Pharmaceutical’s resmetirom (selective thyroid hormone receptor-β agonist; THR-Beta agonist) poised to be the first to market for NASH, there is still a significant unmet need for patients in the later stages of the disease, particularly those with decompensated cirrhosis (DC) due to NASH. This article will provide an overview of the late-stage pipeline candidates that are in development for patients with DC due to NASH and analyse their different mechanisms of action (MOAs).
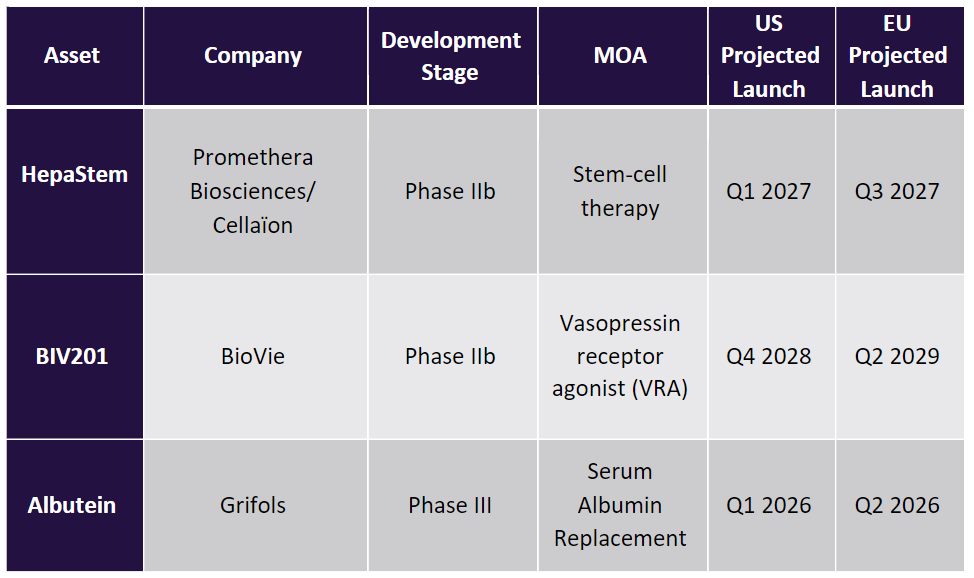
As seen in the table above, there are currently three pipeline agents being developed for the treatment of NASH patients with DC: Promethera Biosciences/Cellaion’s HepaStem (heterologous human adult liver progenitor cells), BioVie’s BIV201 (continuous infusion terlipressin), and Grifols’s Albutein (albumin-human injection). Albutein appears to be on track to be the first therapeutic approved for DC, with a projected US launch date in Q1 2026. Albutein, a serum albumin replacement therapy, is largely a symptomatic treatment to improve fluid homeostasis, thereby reducing hemoconcentration and blood viscosity, as well as a transport protein that binds naturally occurring, therapeutic, and toxic materials in circulation. Additionally, albumin is essential for maintaining the oncotic pressure in the vascular system, which prevents the formation of ascites due to leaked fluid from interstitial spaces into the peritoneal cavity. However, clinical research has shown that despite the extensive use of albumin infusions in patients with cirrhosis to improve renal function and facilitate the elimination of ascites, data suggests that the observed benefits are very modest and limited only to patients with slightly impaired renal function who respond to conventional therapy.
BioVie’s BIV201 is projected to be marketed in the US in Q4 2028. Similar to albutein, BIV201 (terlipressin diacetate) is being evaluated as a continuous infusion in addition to standard of care (SoC) (diuretics and therapeutic paracentesis) for the reduction of ascites and complications in adult patients with refractory ascites secondary to DC. Unlike Albutein, BIV201 is a vasopressin receptor agonist (VRA). Through this MOA, the drug binds to vasopressin receptors and reduces the portal vein pressure (portal hypertension) and increases mean arterial pressure (MAP) to down-regulate the excessive salt and water retention that leads to ascitic fluid buildup. As such, BIV01 may prove to be more effective for the treatment of ascites, as it works to limit the underlying cause of disease progression. In March 2023, BioVie announced the pause of patient enrollment in the company’s Phase IIb clinical trial evaluating BIV201 for the treatment of refractory ascites due to overwhelmingly positive data observed from the first 15 patients in the study. According to the data, BIV201 led to statistically significant decreases in ascites accumulation at Week 4 and Week 12 and in key DC biomarkers. Following the positive data, BioVie disclosed plans to initiate conversations with the FDA before the commencement of the pivotal Phase III trial to accelerate BIV201’s market entry.
Promethera Biosciences/Cellaion’s HepaStem, which is projected to hit the US market in Q1 2027, is a cell therapy that addresses different components of NASH disease progression. Consisting of liver-derived mesenchymal stem cells (MSC), HepaStem is administered intravenously without the need for immunosuppressants and enters the liver via the bloodstream, where it then targets multiple disease pathways. According to Promethera, HepaStem can reduce tissue fibrosis and promote the restoration of liver function by lowering inflammation, deactivating stellate cells, and reducing fibrosis. Currently, there is limited data regarding the therapeutic benefit of HepaStem in patients with DC due to NASH. Results from the Phase IIb trial are expected to be presented at the American Association for the Study of Liver Diseases (AASLD) 2023, scheduled to take place from 10 to 14 November. The trial, which concluded in January 2023, evaluated HepaStem in patients who were recently diagnosed (up to one week) with acute-on-chronic liver failure (ACLF) grade 1 or 2 on top of SoC, and for whom the diagnosis was not resolved on the day of infusion.
Of the three agents currently in late-stage development for DC due to NASH, HepaStem appears to be the most promising agent as it is the only therapeutic among the three that is being investigated with the purpose of restoring liver function and reverse decompensation. However, BIV201 and Albutein may be favourable options as bridge therapies to increase survival time for NASH patients awaiting a liver transplant. For context, most patients with DC typically have an average survival period of two years without transplantation, with the waiting period for a liver transplant varying from as short as less than 30 days to more than five years. Notably, as the NASH market continues to see approvals for agents focusing on cirrhosis improvement and liver regeneration that are indicated for patients in the earlier stages of NASH, the need for a treatment option for DC due to NASH will decrease as fewer patients develop DC. However, until that point is reached, it remains to be seen whether or not HepaStem, BIV201, and Albutein will perform and gain approval.
<!– GPT AdSlot 3 for Ad unit 'Verdict/Verdict_In_Article' ### Size: [[670,220]] —
!– End AdSlot 3 –>
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- ChartPrime. Elevate your Trading Game with ChartPrime. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://www.clinicaltrialsarena.com/comment/leading-players-in-decompensated-cirrhosis-due-to-nash/
