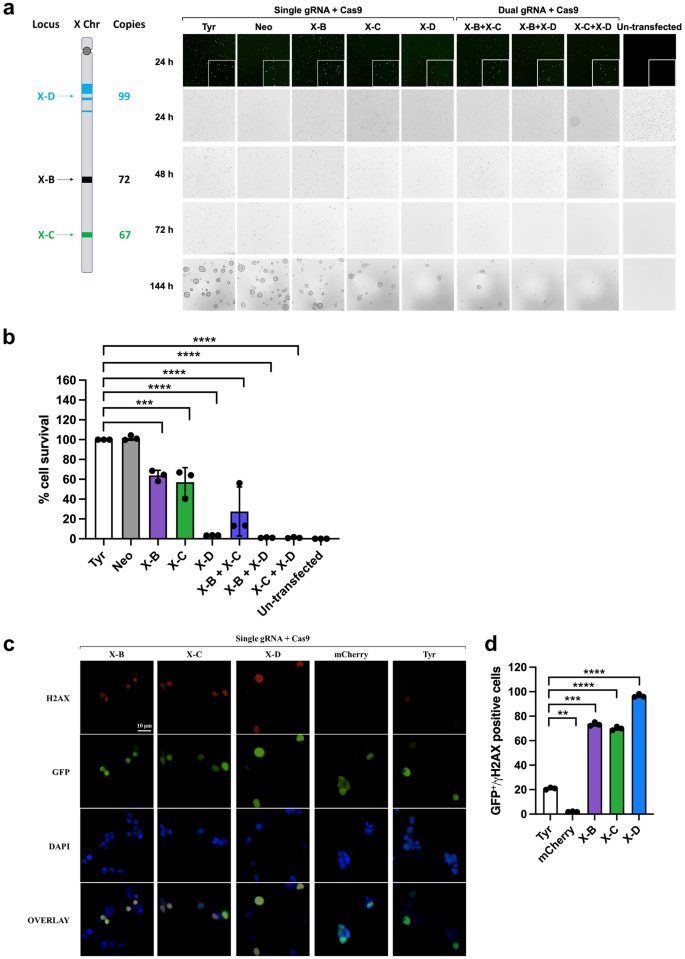
In vitro gRNA-mediated cutting of the X chromosome
We initially tested X-shredder gRNAs in mouse embryonic stem (mES) cells to determine the efficiency of X chromosome elimination. X-B gRNA targets 72 sites spread over ~ 1.7 kb, X-C gRNA targets 67 sites spread over ~ 2.1 Mb, and X-D gRNA targets 99 sites spread over ~ 22 Mb on mouse X chromosome. Mouse ES cells co-transfected with a GFP-expression plasmid and the gRNA constructs (X-B, X-C, X-D13), followed by puromycin selection, showed significant cell death compared to control gRNAs that target a single copy autosomal gene (Tyrosinase) or without a genomic target (Neomycin), supporting X chromosome shredding activity (normalised to Tyr 100%, X-B 63.8 ± 5.3%, X-C 57.1 ± 14.7%, X-D 3.4 ± 0.12%, X-B+X-C 27.5 ± 24.8%, X-B+X-D 1.1 ± 0.3%, X-C+X-D 1.1 ± 0.59%) (Fig. 1a, b). Viability was assessed at 144h post-transfection as the peak growth of mES cells is observed 120–144 h after cell seeding. We found that X-D resulted in the greatest level of cell death (> 95%) compared with X-B (> 35%) and X-C (> 40%). Dual gRNA expression (X-B+X-C, X-B+X-D, and X-C+X-D) further increased cell lethality. Sequencing of target regions revealed indels at single cut sites in remaining cells, confirming DNA cleavage at target sites (Supplementary Fig. 1). We further validated DNA cutting activity of gRNAs by scoring DNA double strand breaks (DSB) detected via gamma-H2AX antibody binding (Fig. 1c, d). Mouse ES cells transfected with X-shredder gRNA showed significantly higher DSBs (3.3–4.6 fold) compared with the tyrosinase gRNA control, further supporting X-shredder gRNA cleavage activity. The mCherry control gRNA does not target mouse genome, consistent with the near absence of DSBs.
Elimination of the X chromosome and creation of DNA double strand break in mouse ES cells by CRISPR/Cas9-mediated gene editing. (a) Schematic of X-B, X-C, and X-D target sites and their copies on the mouse X chromosome and representative brightfield microscopy images of mouse ES cells 24 h, 48 h, 72 h, and 144 h after transfection and puromycin selection. Co-transfection with a GFP-expression plasmid was performed to assess transfection efficiency (top). mES cells grow as dome shaped colonies which are apparent at the 144h timepoint. Scale bar = 100 µM. (b) Cell survival percentage at 144 h post-transfection of X-shredder gRNAs (n = 3; Bars show mean ± SD, one way ANOVA with Sidak’s multiple comparison test). (c) Representative immunofluorescence images of mouse ES cells 24 h after transfection. Scale bar = 10 µM. (d) Average numbers of GFP/gamma-H2AX double positive cells (n = 3). Bars show mean ± SD, one way ANOVA with Sidak’s multiple comparison test.
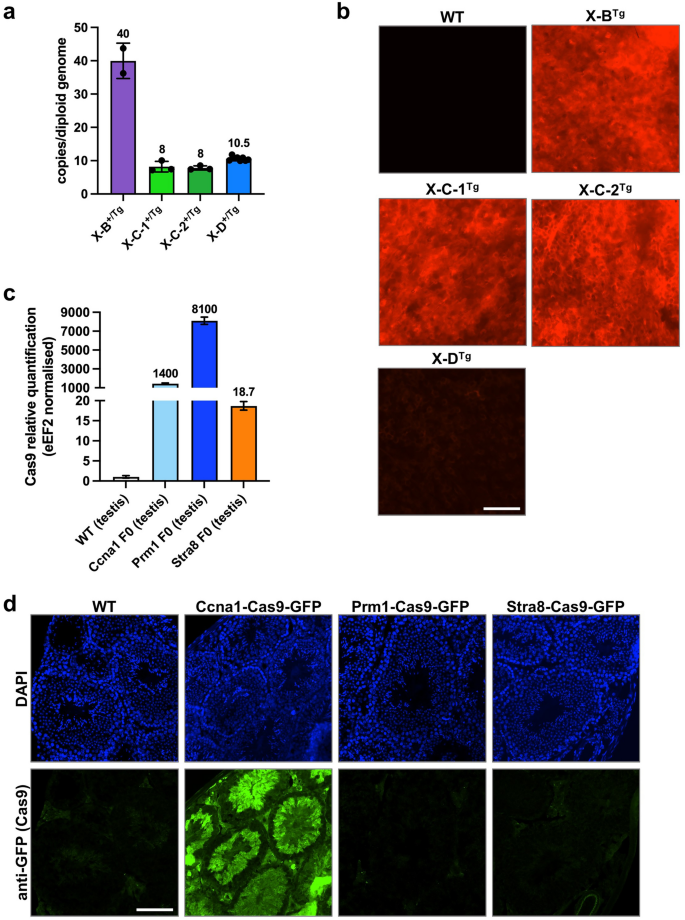
X-shredder transgenic mouse generation
To assess X-shredding activity in vivo, we employed a ‘split drive’ system. gRNA- and Cas9-expressing mouse lines were generated separately and then crossed, resulting in ubiquitous gRNA and male germline-specific Cas9 expression. All transgenes were randomly integrated into the genome using pronuclear injection. We established Cas9 expression lines using the previously validated germline promoter sequences of Stra8, Ccna1, and Prm115,16,17,18,19 which are active in pre-meiotic, meiotic (leptotene-zygotene) and post-meiotic spermatocytes, respectively. Cas9 was linked to an EGFP reporter using a P2A self-cleaving peptide (Supplementary Fig. 2a;6). X-B, X-C, and X-D gRNA sequences were driven by the U6 promoter. A CMV mCherry fluorescent reporter cassette was also included to facilitate identification of transgene carriers (Supplementary Fig. 2b). We generated single lines for X-B and X-D which contained 40 and 10 transgene copies, respectively (Fig. 2a). Two independent X-C lines, X-C-1 and X-C-2 were generated, both of which carried eight copies of the transgene (Fig. 2a). All lines were positive for mCherry fluorescence as determined by ear skin biopsies, although, X-D mice had much lower expression compared with the other lines (Fig. 2b).
Characterisation of X shredder-mCherry and germline promoter-Cas9 transgenic mice. (a) Transgene copy number of hemizygous X-B (n = 2), X-C-1 (n = 3), X-C-2 (n = 3) (two founder lines), and X-D (n = 10) mice. Bars show mean ± SD. (b) Fluorescence imaging performed on ear skin punch biopsies for the transgenic mouse lines described in A. showing representative mCherry signal (red) with a WT mCherry negative control. n = > 30 per genotype. Scale bar = 200 µM. (c) Expression of Cas9 RNA in testis isolated from Ccna1-, Prm1-, and Stra8-Cas9 transgenic mouse lines. Expression is normalised to eEF2 and WT testis indicates background Cas9 detection in this assay. n = 1 per genotype. (d) Representative IF of Cas9-GFP expression (green) in the testis of Ccna1-Cas9-GFP transgenic mice. GFP signal was amplified by staining with an anti-GFP antibody while DAPI nuclear staining (blue) shows tubule structures. n = 2 per genotype, mice 8–24 weeks of age. Scale bar = 100 µM.
RNA harvested from the testis of Cas9-EGFP transgenic mouse lines was used for qRT-PCR to determine Cas9 expression levels. The Ccna1-Cas9-EGFP and Prm1-Cas9-EGFP lines expressed high levels of Cas9 mRNA while lower transgene expression was detected in Stra8-Cas9-EGFP testes (Fig. 2c). However, we could only detect EGFP fluorescence in testis sections of Ccna1-Cas9-EGFP mice, where it localised to the tubules and was expressed in late spermatocytes (D and m) to elongating spermatids (1–14 of spermiogenic cycle) (Fig. 2d, Supplementary Fig. 2C). We therefore focussed mainly on the Ccna1-Cas9 line for in vivo experiments.
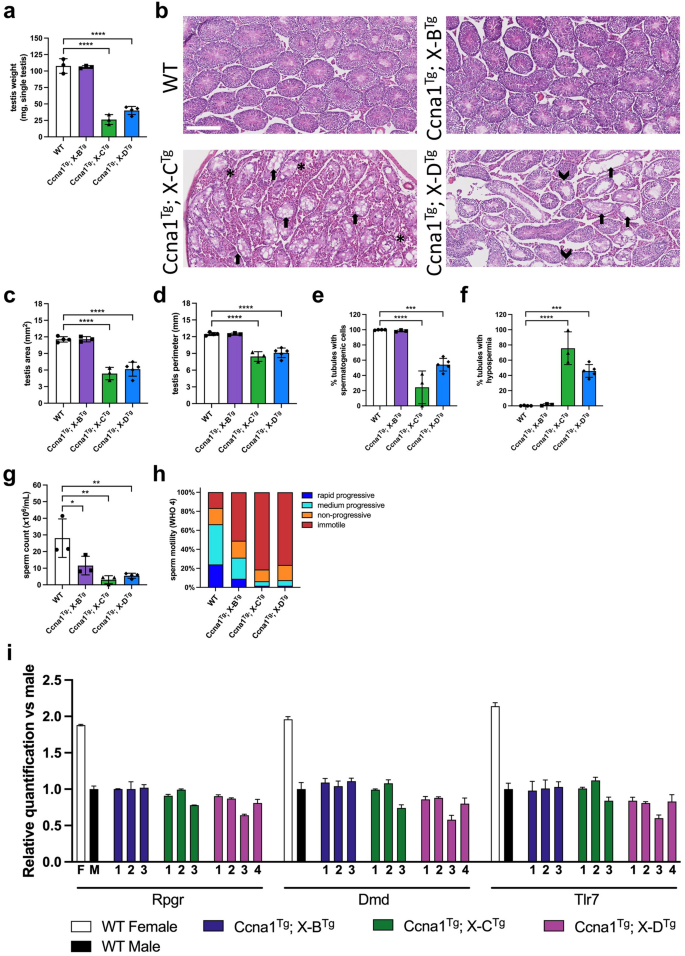
Characterisation of gRNA; Cas9 double transgenic mice
We generated double transgenic progeny expressing an X-shredding gRNA together with Cas9 by mating X-B, X-C (two lines, X-C-1 and X-C-2), or X-D lines with Ccna1-Cas9-EGFP. The weight of Ccna1Tg; X-BTg testis were comparable to wild type (WT), while both Ccna1Tg; X-CTg lines and Ccna1Tg; X-DTg mice had significantly smaller testis (Fig. 3a). Histological analysis showed relatively normal tubule structure in Ccna1Tg; X-BTg mice while both Ccna1Tg; X-CTg lines and Ccna1Tg; X-DTg mice exhibited disrupted tubule architecture (Fig. 3b). Two-dimensional area and perimeter of Ccna1Tg; X-CTg and Ccna1Tg; X-DTg testes were also significantly decreased compared with WT (Fig. 3c and d, respectively). Tubules either lacking or with few spermatogenic cells were common in Ccna1Tg; X-CTg and Ccna1Tg; X-DTg testes (Fig. 3b, e, f), as were the presence of tubule vacuoles. All double transgenic lines showed significantly reduced sperm concentrations and dramatically reduced sperm motility fitness compared with WT (Fig. 3g, h).
Functional assessment of Ccna1-Cas9Tg; X-shredderTg double transgenic mice. (a) WT, Ccna1Tg; X-BTg, Ccna1Tg; X-CTg, and Ccna1Tg; X-DTg ex-breeder males were sacrificed, testis removed and weighed on a fine balance. (b) H&E staining of representative testis sections from mice used in A. showing disrupted tubule architecture in Ccna1Tg; X-CTg and Ccna1Tg; X-DTg mice. Arrows indicate empty tubules. Arrow heads indicate vacuoles. Asterisks indicate areas with an abundance of Leydig cells. Scale bar = 250 µM. Quantification of two-dimensional area (c) and perimeter size (d) from sections. (e) Enumeration of the frequency of tubules containing spermatogenic cells. (f) Quantification of the frequency of empty tubules. (g) Sperm isolated from the epididymis were enumerated using a Sperm Class Analyser. (h) Sperm motility was measured according to WHO 4 metrics. Mean motility values from mice shown. (i) X chromosome dosage was assessed by qPCR using sperm DNA isolated from WT, Ccna1Tg; X-BTg, Ccna1Tg; X-CTg, and Ccna1Tg; X-DTg mice. Male mouse DNA containing one X chromosome was used as the reference. Mean ± SD of triplicates for each mouse is shown. (a) and (c–h) n = 3 for WT, Ccna1Tg; X-BTg, Ccna1Tg; X-CTg, and n = 4 Ccna1Tg; X-DTg. Bars show mean ± SD. Mice 21–67 weeks of age. (a), (c–i) show X-C-1Tg and X-C-2Tg data combined. Bars show mean ± SD, one way ANOVA with Sidak’s multiple comparison test.
To investigate if disproportionate X chromosome sperm loss was occurring in vivo, we isolated sperm from the vas deferens and cauda epididymis of WT and double transgenic male mice and measured X chromosome dosage by qPCR using three genes spanning the X chromosome (Rpgr, DMD, and Tlr7). Interestingly, Ccna1Tg; X-DTg male 3 and Ccna1Tg; X-CTg male 3 had lower gene dosage at all three loci (Fig. 3i). The former was particularly striking with only ~ 60% X chromosome dosage detected. Ccna1Tg; X-DTg males 1, 2 and 4 also had lower dosage across 2 of the 3 loci. In contrast, Ccna1Tg; X-BTg males had normal X chromosome dosage. These data suggest that disproportionate X chromosome loss is occurring in vivo when the X-C and X-D gRNA and Cas9 are expressed in the male germline, although the effect is variable and incompletely penetrant.
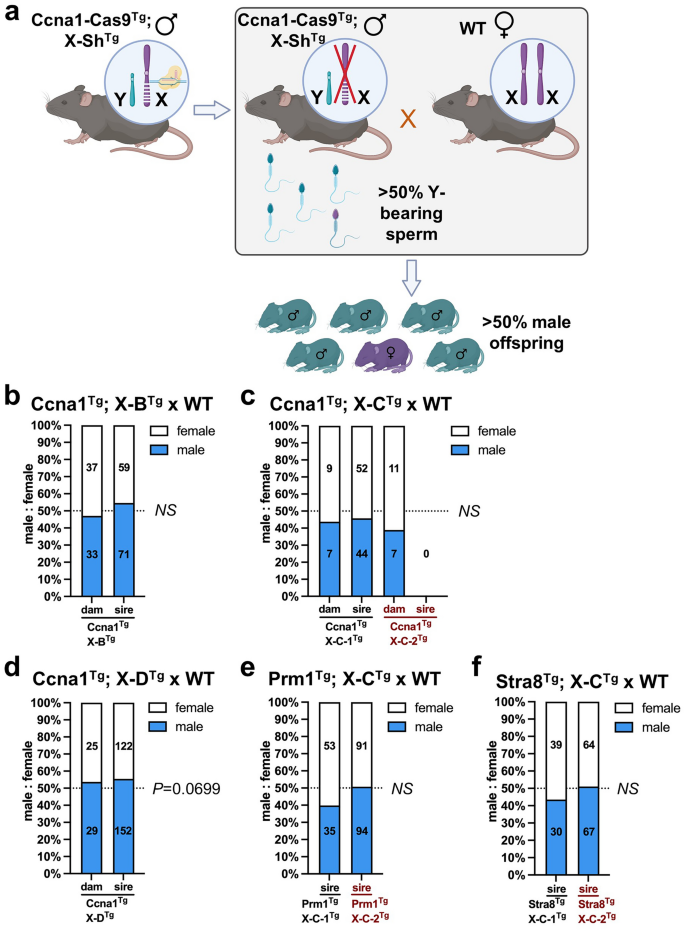
Biased generation of male offspring is not observed in experimental matings
To investigate male offspring bias, we mated Ccna1-Cas9Tg; X-shredder-gRNATg males with WT females and assessed the proportion of male and female progeny (Fig. 4a). As negative controls, we also mated genetically equivalent littermate transgenic females with wild type males (spermatogenesis promoters should not be active in females). Progeny from Ccna1Tg; X-BTg and Ccna1Tg; X-CTg sires from both X-CTg lines did not show significant deviation from a 50:50 male:female ratio (Fig. 4b, c). Similarly, Cas9 expression driven by Prm1 or Stra8 did not significantly alter the male:female ratio when used in combination with the both X-CTg lines (Fig. 4e, f). Interestingly, Ccna1Tg; X-DTg males sired an excess of male progeny (152 males versus 122 females) but this failed to reach statistical significance (P = 0.0699; Fig. 4d). To further assess male bias, we performed in vitro fertilisation using sperm isolated from a Ccna1Tg; X-DTg male. Following generation of zygotes, blastocysts were allowed to develop, DNA was extracted, and the ratio of male to female blastocysts was enumerated. No significant difference in the male:female ratio was observed, confirming that significant loss of X-bearing sperm was not generally occurring in double transgenic males (Supplementary Fig. 3a, b).
Transgenic mice co-expressing X-shredder gRNAs and germline promoter-Cas9 did not generate altered male bias. (a) Schematic showing the method to determine if male bias was occurring in offspring of germline promoter-Cas9Tg; X-shredderTg male × WT females (created with Biorender.com). Control crosses consisted of germline promoter-Cas9Tg; X-shredderTg females × WT males. Enumeration of female and male pups from: (b) Ccna1Tg; X-BTg × WT matings. Control matings = 11 plugs from three transgenic females with 10 litters, experimental matings = 29 plugs from three transgenic males with 22 litters. (c) Ccna1Tg; X-CTg × WT matings (two independent X-CTg founder lines). Ccna1Tg; X-C-1Tg control matings = 4 plugs from one transgenic female with 3 litters, Ccna1Tg; X-C-1Tg experimental matings = 28 plugs from one transgenic male with 15 litters. Ccna1Tg; X-C-2Tg control matings = 3 plugs from one transgenic female with 3 litters, Ccna1Tg; X-C-2Tg experimental matings = 29 plugs from two transgenic males with 0 litters. (d) Ccna1Tg; X-DTg × WT matings. Control matings = 7 plugs from three transgenic females with 8 litters, experimental matings = 109 plugs from three transgenic males with 48 litters. (e) Prm1Tg; X-CTg × WT matings (two independent X-CTg founder lines). Prm1Tg; X-C-1Tg experimental matings = 16 plugs from one transgenic male with 14 litters. Prm1Tg; X-C-2Tg experimental matings = 42 plugs from three transgenic males with 27 litters. (f) Stra8Tg; X-CTg × WT matings (two independent X-CTg founder lines). Stra8Tg; X-C-1Tg experimental matings = 16 plugs from one transgenic male with 10 litters. Stra8Tg; X-C-2Tg experimental matings = 20 plugs from three transgenic males with 18 litters. (b–f) Numbers in bars indicate number of female pups (white) and male pups (blue) combined from litters of the same genotype. Chi squared test.
Fertility defects in gRNA; Cas9 double transgenic mice
Given the small testes and low sperm count in some double transgenic males, we compared litter size and plug-to-pregnancy rates with controls (double transgenic females, where available) and historical non-transgenic pregnancy rates from WT mice maintained in our animal facility. Males remained in the cage with two females until the latter were plugged which averaged 1–7 days. No significant changes in litter size were seen in double transgenic mice compared with single transgenic controls kept under the same conditions (Supplementary Fig. 4a-e ). Ccna1Tg; X-BTg (3 males), Prm1Tg; X-C-1Tg (1 male), Prm1Tg; X-C-2Tg (3 males), Stra8Tg; X-C-1Tg (1 male) and Stra8Tg; X-C-2Tg (2 males) double transgenic males sired litters at normal rates (Supplementary Fig. 4a, c, d). The Ccna1Tg; X-C-1Tg line (3 males) exhibited normal fertility in contrast to the Ccna1Tg; X-C-2Tg line which failed to have any litters (Supplementary Fig. 4b). Ccna1Tg; X-DTg double transgenic males varied in their ability to sire pups, with 2/5 males exhibiting normal fertility, while 3/5 had reduced fertility (Supplementary Fig. 4e).
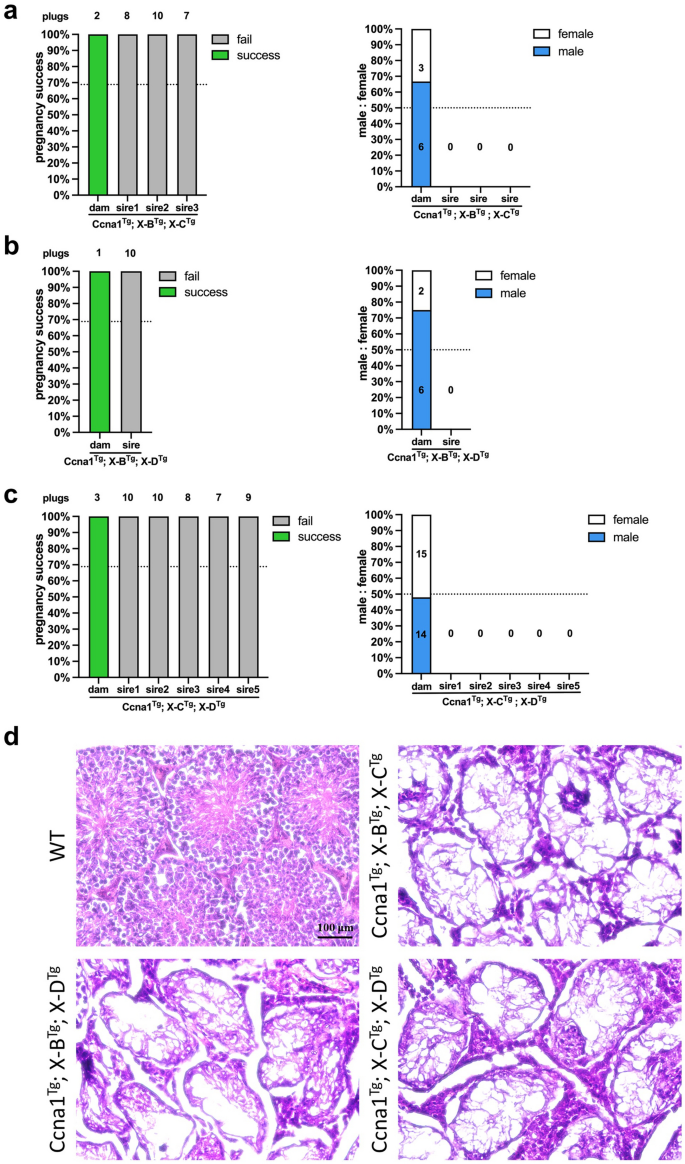
Triple transgenic X-shredder mice are infertile
Given that the gRNA target sites for X-B, X-C and X-D are relatively localised on the X chromosome, we reasoned that expressing two different gRNAs targeting independent repeats would increase the likelihood of generating irreparable damage, resulting in X chromosome loss. Therefore, we generated Ccna1Tg; X-BTg; X-CTg (Fig. 5a, X-C-1 and X-C-2 lines, 3 males), Ccna1Tg; X-BTg; X-DTg (Fig. 5b, 1 male), and Ccna1Tg; X-CTg; X-DTg (Fig. 5c, X-C-1 and X-C-2 lines, 5 males) triple transgenic males which were mated with WT females to assess male offspring bias. However, triple transgenic males were unable to sire litters with WT dams despite successful plug generation. Female triple transgenic mice had expected pregnancy success (Fig. 5a–c, left) and generated litters with normal male:female ratios (Fig. 5a–c, right). Histological examination of the testis from triple transgenic males revealed severe disruption to tubule architecture and lack of any spermatogenic cells, consistent with azoospermia and their inability to sire offspring (Fig. 5d).
Functional assessment of Ccna1-Cas9Tg; X-shredderTg triple transgenic mice. Pregnancy success as determined by plug and successful pregnancy counts (left) and enumeration of male and female pups (right) from: (a) Ccna1Tg; X-BTg; X-CTg × WT matings. Control matings = 2 plugs from one transgenic female with 2 litters, experimental matings = 25 plugs from three transgenic males with zero litters. (b) Ccna1Tg; X-BTg; X-DTg × WT matings. Control matings = 1 plug from one transgenic female with 1 litter, experimental matings = 10 plugs from one transgenic male with zero litters. (c) Ccna1Tg; X-CTg; X-DTg × WT matings. Control matings = 3 plugs from one transgenic female with 3 litters, experimental matings = 44 plugs from five transgenic males with zero litters. (d) H&E staining of representative testis sections from mice used in (a), (b) and (c). Mice 15–17 weeks of age. Scale bar = 100 µM.
Molecular characterisation of in vivo X chromosome cleavage
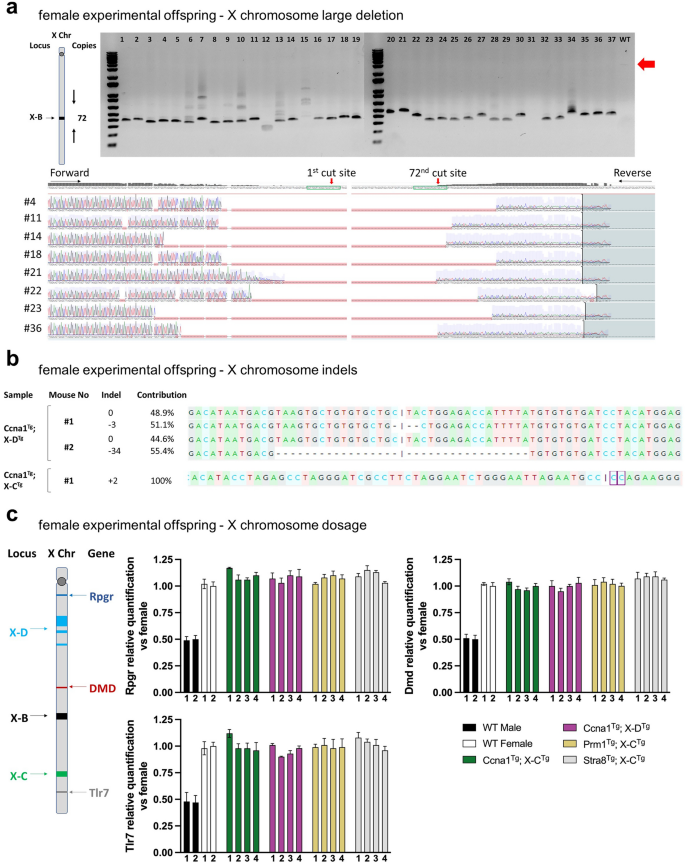
To better understand the cleavage activity of X-shredder gRNAs during spermatogenesis, we looked for indels at gRNA target sites in female offspring from double transgenic sires. We focussed initially on Ccna1Tg; X-BTg offspring, as the target repeats are contained within a localised 1,800 bp region that is amenable to PCR amplification (Fig. 6a). Analysis of the X-B target site from 37 randomly selected females showed large deletions of approximately 1750 bp (Fig. 6a). Sequencing of the PCR products and alignment to the WT sequence confirmed the presence of large deletions in all the sequenced samples, indicating robust cleavage of the X-B target site (Fig. 6a). As the repeat sequences targeted by X-C and X-D span much larger regions, a single target site was selected for a similar analysis of 13 randomly selected females from Ccna1Tg; X-C-1Tg sires and 31 from Ccna1Tg; X-DTg sires. One sample from Ccna1Tg; X-C-1Tg sires and 2 samples from Ccna1Tg; X-DTg sires had indels at the selected single cut site showing a low rate of indels (Fig. 6b). No target site cutting activity was observed in 44 randomly selected females from Prm1Tg; X-CTg (both X-C-1 and X-C-2 lines), and 14 from Stra8Tg; X-C-1Tg sires (Supplementary Fig. 5c). Sperm from Prm1Tg; X-CTg and Stra8Tg; X-CTg sires was analysed for indels but none were detected (Supplementary Fig. 5a. One male (out of 20 samples) from a Ccna1Tg; X-DTg sire had an indel (Supplementary Fig. 5b), presumably generated by Cas9/gRNA carryover.
Molecular evidence of X chromosome-targeted Cas9 activity from female offspring of experimental matings. (a) Schematic of X-B gRNA target region showing number of cuts and distribution of cut sites on the mouse X chromosome. PCR across the X-B-targeted cut site region on the X chromosome of pups generated from Ccna1Tg; X-BTg male × WT female matings. Expected uncut DNA amplicon size is ~ 3000 bp. Smaller products in the DNA gel indicate deletions. Associated Sanger sequencing of X-B gRNA target site from selected samples demonstrates efficient deletion of the region containing repeat sequences. (b) Sanger sequencing of DNA from female pups of Ccna1Tg; X-DTg male × WT female matings (top) and Ccna1Tg; X-CTg male × WT female matings (bottom) of X chromosome target sites showing the presence of small indels. (c) Schematic of X-B, X-C, and X-D target sites and Rpgr, DMD, Xist, and Tlr7 gene loci on the mouse X chromosome. X chromosome dosage qPCR was performed on genomic DNA isolated from female pups of germline promoter-Cas9Tg; X-shredderTg male × WT female matings. Expression of Rpgr, DMD, Xist and Tlr7 was normalised to WT female genomic DNA.
Finally, as XO mice are female and viable, we investigated the possibility that female offspring of double transgenic sires did not inherit a paternal X chromosome. X chromosome gene dosage was performed by qPCR analysis of Rpgr, DMD, and Tlr7 in randomly selected female pups from matings of Ccna1Tg; X-DTg sire, Ccna1Tg; X-CTg sire, Prm1Tg; X-CTg sire or Stra8Tg; X-CTg sires with WT females. No reduction in X chromosome dosage was detected (Fig. 6c) indicating that paternal X chromosome transmission was not compromised.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-024-63706-4