They published their work on Dec. 20 in Energy Material Advances.
They published their work on Dec. 20 in Energy Material Advances.
“Economic, efficient, and safe hydrogen storage methods play a crucial role in exploiting hydrogen energy, reducing carbon emissions, and improving the utilization efficiency of renewable clean energies,” said paper author Jianxin Zou, professor in National Engineering Research Center of Light Alloys Net Forming & State Key Laboratory of Metal Matrix Composites. “Solid-state hydrogen storage in hydrides has been considered as a promising hydrogen storage technology. Although the industrial application of solid-state hydrogen storage technologies with metal hydride is still in the stage of an attack.”
“Take Magnesium-based hydrides as an example, Magnesium is the eighth most abundant element in Earth’s crust, and low-cost, with excellent operation safety and environmentally friendly,” Zou said. “Magnesium-based hydrides are attractive candidates for large-scale hydrogen energy storage systems due to their high hydrogen storage densities, good cyclic performance, and high abundance of Mg on earth.”
“The implementation of MgH2 as a hydrogen-storage medium has long been restricted by two dominating intrinsic challenges.”
Zou explained that the first obstacle is the high thermodynamic stability (ΔH = 74.7 kJ mol−1 H2) resulting in the high decomposition temperature of MgH2. Another obstacle is the rather sluggish hydrogen ab/de-sorption kinetics originating from high H2 dissociation energy barrier, slow hydrogen diffusion rate in MgH2 bulk.
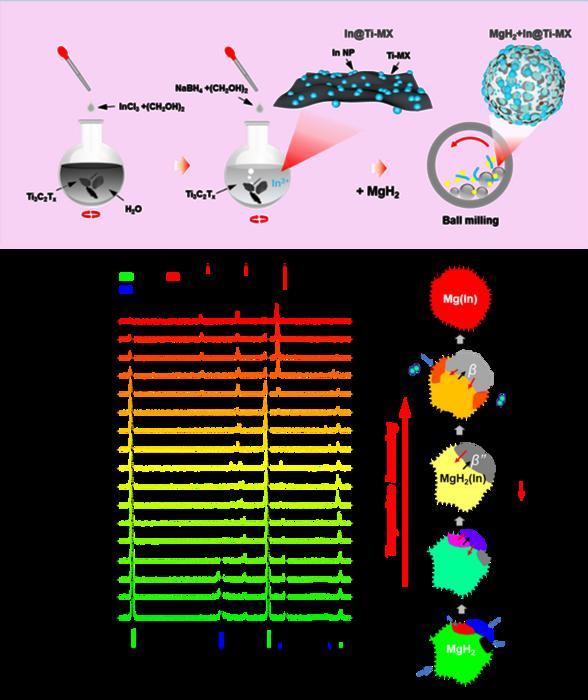
To improve hydrogen storage performance of MgH2/Mg, many efforts have been made, such as catalyst doping, nano-crystallization, alloying, destabilization. And achieving dual regulation of the kinetics and thermodynamics of MgH2 is essential for the practical applications. Previous works proved that introducing catalysts into Mg/MgH2 could accelerate the hydrogen de-/absorption kinetics obviously. Among all the catalytic compounds, MXenes-based catalysts have attracted significant attention owing to the unique two-dimensional structure and the component tunability of MXenes. However, similar to common catalysts, most MXenes-based catalysts, such as Ti3C2 MXenes and Ni@Ti-MX, could not alter the thermal stability of MgH2/Mg, resulting in high desorption temperature at 1 bar hydrogen pressure and complex heat management processes for practical applications.
Alloying is a prominent approach to improve the thermodynamic and kinetic properties of Mg-based hydrogen storage materials. It’s worth noting that using solid solution alloys can improve the thermodynamics of MgH2 without significant capacity loss. Indium, cadmium, silver, etc. have been proved to destabilize the thermodynamics of MgH2 by forming a solution with Mg. Because of the high solubility of In in Mg (up to 10 at%), Mg-In system exhibits the best improvement in thermodynamics. Zou and his team reviewed the latest works, comparing the enhancement in the kinetics and thermodynamics of previous works to identify areas that future research should focus and strategies.
“MXene not only possesses excellent catalytic effects, but also provides nanoconfinement ability via its two-dimensional layered structure, which can effectively improve both the kinetic property and the cycling stability of the Mg based composites. On the other hand, In alloying has been demonstrated to be an efficient way to achieve thermodynamic destabilization of the Mg/MgH2 system,” Zou said. “In this paper, we aim to combine the advantages of MXenes and Mg(In) solid solution in the modification of MgH2/Mg to simultaneously alter the thermodynamics and kinetics of Mg-based hydrogen storage materials.”
“Results showed that both the thermodynamics and kinetics of Mg-In-Ti hydrogen storage system have been optimized. The enhanced de/hydrogenation properties of MgH2 catalyzed by In@Ti-MX and corresponding mechanisms have been systematically probed and elaborated,” Zou said.
It should be emphasized that the kinetic and thermodynamic properties of Mg-In-Ti hydrogen storage system is still far from the requirements for the on-board applications. There is still a long way to go for the commercial-scale production and practical application of such intriguing materials. Zou reviewed, breaking through the barrier of operating temperatures is still the focus of the future work.
“Although MgH2 has been extensively studied as one of the most promising solid-state hydrogen storage materials, its application in other energy fields has attracted little attention. Considering the low cost and unique phase transformation behavior, we expect to see a surge in the application of nanostructured Mg-based hydrogen storage materials in various energy fields, such as energy storage of renewable energy.”
Jianxin Zou is also affiliated with Shanghai Engineering Research Center of Mg Materials and Applications & School of Materials Science and Engineering, and Shanghai Key Laboratory of Hydrogen Science & Center of Hydrogen Science, Shanghai Jiao Tong University. Other contributors include Wen Zhu, Li Ren, Yinghui Li, Chong Lu, Xi Lin, Qiuyu Zhang, Xue Yang, Zhigang Hu, Tao Cheng and Yingyan Zhao.
The following authors have additional affiliations: Xue Yang and Tao Cheng, Sinopec Research Institute of Petroleum Processing Co., LTD.
The National Key R&D Program of China (No. 2022YFB3803700) and the National Natural Science Foundation (No. 52171186) supported this work.
###
Reference
Authors: WEN ZHU, LI REN, YINGHUI LI, CHONG LU, XI LIN, QIUYU ZHANG, XUE YANG, ZHIGANG HU, TAO CHENG, YINGYAN ZHAO , AND JIANXIN ZOU
Title of original paper: In situ High-Energy Synchrotron X-ray Studies in Thermodynamics of Mg-In-Ti Hydrogen Storage System
Journal: Energy Material Advances
DOI: 10.34133/energymatadv.0069
Affiliations:
1National Engineering Research Center of Light Alloys Net Forming & State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240, PR China.
2Shanghai Engineering Research Center of Mg Materials and Applications and School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai, 200240, PR China.
3Shanghai Key Laboratory of Hydrogen Science and Center of Hydrogen Science, Shanghai Jiao Tong University, Shanghai, 200240, PR China.
4Sinopec Research Institute of Petroleum Processing Co., Ltd., Beijing, 100083, PR China.
About Prof. Zou:
Prof. Jianxin Zou is a professor in the School of Materials Science and Engineering of Shanghai Jiao Tong University, deputy director of the Center of Hydrogen Science of Shanghai Jiao Tong University, a Youth Changjiang Scholar of the Ministry of Education, and the chief scientist of the National Key Research and Development Program. Prof. Zou has published more than 160 papers in peer reviewed journals, like Science, Advanced Materials, Advanced Energy Materials, Nano-micro Letters, ACS Nano, et al., with more than 6100 citations (H factor = 45). He has won the Award of “Innovative Product of the Year” of the International Mg Society in 2021, the Innovation Prize of the Non-ferrous Metal Society (youth group) in 2022, the 2023 IMA Future Technologies Award, etc. He was admitted as a Fellow of IAAM in recognition for his contribution to hydrogen energy in 2023.
Journal
Energy Material Advances
DOI
10.34133/energymatadv.0069
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
In situ High-Energy Synchrotron X-ray Studies in Thermodynamics of Mg-In-Ti Hydrogen Storage System
Article Publication Date
20-Dec-2023
COI Statement
The authors declare that they have no competing interests.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://bioengineer.org/in-situ-characterization-reveals-different-dehydrogenation-pathways-in-mgh2/
