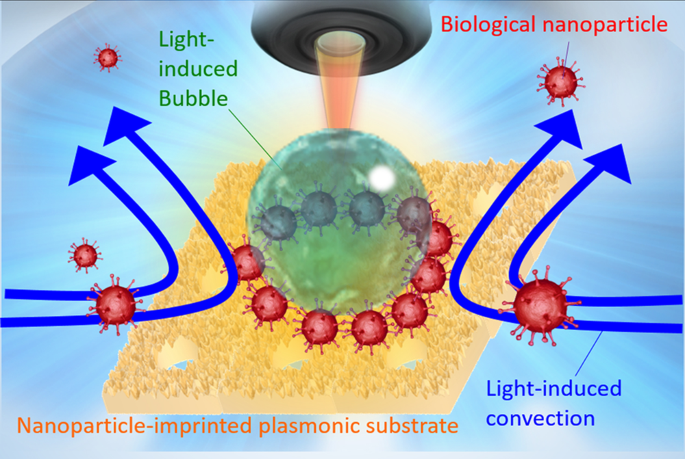
Production of the nanoparticle-imprinted plasmonic substrate (NPI-PS)
A glass-bottom dish (GBD, D11130H; Matsunami Glass, Osaka, Japan) consisting of a glass substrate surrounded by a 1.4-cm diameter cylindrical plastic wall was used to fabricate the periodic bowl-shaped structure of the NPI-PS (Fig. 2b). A thin gold film (thickness: 50 nm) was deposited on a GBD using an ion sputtering system (E-1010; Hitachi, Tokyo, Japan). Carboxyl group-modified polystyrene particles (09836; Polysciences, Inc., Warrington, PA, USA) with an average diameter of 500 nm were self-assembled on a thin gold film and used as templates for the bowl-shaped NPI-PS. The polypyrrole covering the mould was applied by pyrrole electropolymerisation (167-05662, Wako Pure Chemical Industries, Ltd., Osaka, Japan) over a self-assembled periodic array of polystyrene particles using platinum wire as a cathode and a gold-coated GBD as an anode. Subsequently, the polystyrene particles were dissolved using chloroform (035-02616; Wako) to obtain a bowl-shaped structure. Finally, a gold thin film was formed again. The NPI-PS structure was observed via scanning electron microscopy (JSM-IT100; JEOL, Japan Electron Optics Laboratory CO., LTD, Tokyo, Japan).
Preparation of virus-mimicking nanoparticles (VMNPs)
The VMNPs were prepared with fluorescent streptavidin-modified beads (30-19-102; Micromod Partikeltechnologie GmbH, Rostock, Germany; undiluted concentration is 1.9 × 1013 particles/mL, Rhodamin B (excitation:552 nm, emission:580 nm) was doped), biotinylated anti-S2 antibody (GTX632604; GeneTex, Irvine, CA, USA; undiluted concentration is 0.285 mg/mL), and S-protein (10549-CV; R&D Systems, Minneapolis, MN, USA; undiluted concentration is 0.5 mg/mL) that were mixed and allowed to stand at 42 °C for 1 h; the solvent was replaced by phosphate buffer (10 mM, pH 6.6) using a dialysis kit (40784; Scienova GmbH, Jena, Germany). For buffer replacement, the buffer was replaced and allowed to stand for 15 min four times. After that, the buffer was replaced and allowed to stand for 1 h, and this was replaced and allowed to stand for 2 h. Then, the obtained buffer was replaced and allowed to stand overnight (16–20 h, temperature was kept at 0 °C). In this replacement process, commercial phosphate buffer (FUJIFILM, 164-27135, pH 6.4) was diluted to 10 mM (pH 6.6). Anti-S1 antibody-modified nanoparticles were similarly mixed with streptavidin-modified beads (01-19-102; Micromod) and anti-S1 antibody (GTX635654; GeneTex; undiluted concentration is 0.285 mg/mL), and incubated at 42 °C for 1 h. The solvent was replaced by phosphate buffer (10 mM, pH 6.6) as described above. Biotinylation of each antibody was performed using a water-soluble biotinylating reagent (A39257; Thermo Fisher Scientific, Waltham, MA, USA). In Fig. S6, Artificial Saliva for pharmaceutical research (BZ108, Biochemazone) was used for investigating the effect of foreign substances, where the VMNPs were dispersed into the mixture of equal volumes of the artificial saliva and the diluted phosphate buffer (10 mM, pH 6.6).
Optical system
Fig. S1 is a schematic diagram of the optical system. Reflectance image visualisation and measurement of reflectance spectra of samples were performed using an optical upright microscope (Eclipse 80i; Nikon, Tokyo, Japan) equipped with halogen and mercury lamps as white light sources and a spectroscope (USB4000 (grating #3) SLIT-25; Ocean Optics, Inc., Dunedin, FL, USA) connected to an optical fibre (core diameter: 50 μm). An 800-nm CW laser (FPLD 0806-100-CS; FiberLabs Inc., Saitama, Japan) was introduced into the sample stage using a backport adapter (00272356001; Sigma Koki Co., Ltd., Tokyo, Japan) for optical condensation. To evaluate the optical response of the substrate material, optical condensation, and spectroscopy were performed using a 40× objective lens (CFI Plan Fluor 40x/0.75 DIC M/N2, NA: 0.75, Cover glass thickness: 0.17, WD: 0.66; Nikon, Japan). For fluorescent imaging, a halogen lump was used for Fig. 2d–g, and a mercury lamp was used for Fig. 3, Fig. 4, Fig. S4, Fig. S6 as illumination light sources, respectively, where green fluorescent images were observed via FITC-A-Basic (Nikon Japan) and red fluorescent images were observed via TRICK-A-Basic (Nikon, Japan) as optical filters. In order to binarising red fluorescent image for effective analysis, brightness was added +40% with PowerPoint in Fig. 3b and Fig. S6a. For reflectance spectrum measurements, a halogen lamp equipped with an optical microscope was used as a white light source; reflectance was calculated with respect to a protected silver mirror (PF10-03- P01-10; Thorlabs, MHI, USA).
Assembly area measurement
The area of the assembled structure of the fluorescence polystyrene particles of 100 nm diameter (16662-10; Fluoresbrite Carboxylate Microspheres (2.5% Solids-Latex), YG Polysciences, Inc., Warrington, PA, USA) by optical condensation was estimated from fluorescence images using NIS-Elements Analysis software (Nikon Instruments Inc., Tokyo, Japan). The emission intensity of the bacteria at the edge of the fluorescence image was designated as the standard value. The area of the luminescence collected at the assembly site near the centre of the bubble was measured (i.e., not the luminescence attached to the outside of the bubble). The estimated number of nanoparticles was determined by dividing the fluorescence area from assembled nanoparticles by the cross-sectional area of each nanoparticle calculated from the average diameter of nanoparticles. In Fig. 3 and Fig. S6, an assembly area of target nanoparticles was evaluated binarising each red fluorescent image with ImageJ35 (Ver. v1.54 f) as following steps; <1> The fluorescence image (RGB) was converted into grayscale image using “Split Channels” (function of ImageJ). Each grayscale image in 8 bits was quantified as luminance by splitting the color (0 for black, 255 for white. Each image was divided into 1280 × 960 pixel). <2> in “Threshold” (function of ImageJ), pixels above the threshold setting 50 (red area in the original color image) were considered as VMNPs. <3> the region beyond the threshold selected in <2> larger than 8 pixels was counted as the sum of fluorescence area of VMNPs using “Analyze Particles” (function of ImageJ). <4> When the weighted area should be calculated, the averaged luminance of selected pixels in <2> (value between 0 and 255) was multiplied with the fluorescence area in <3 >, where the product was finally divided by 255.
Theoretical calculations
The experimentally observed optical response of the NPI-PS was compared with solutions obtained from theoretical calculations based on FDTD simulations using the commercial software Ansys Lumerical FDTD Solutions (Ver. 2022 R1.4, Ansys Inc., USA, Pennsylvania). The SEM images were used to create a structural model for the simulation. Due to the periodicity of the substrate structure, periodic boundary conditions were used for the lateral boundaries (The width for x direction is set to 500 nm, and the width of y direction is set to 866.025 nm). The refractive index of the nanoparticles was assumed to be constant as 1.59, referring to the PRODUCT CATALOG of Polysciences 2022/2023. The wavelength dependence of real and imaginary parts of the refractive index of gold film, polypyrrole film and glass substrate were extracted from the previous literatures37,38,39, where experimental data in the literature were fitted to obtain the dielectric function for polypyrrole in the calculation using FDTD Solutions within the wavelength region of 500–800 nm (Fig. S9)38.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s44328-024-00004-z
