Animal models have shown that cardiomyocytes can indeed regenerate. However, animal studies are not always directly transferable to a human setting. Thus, it is essential to assess regenerative processes in human heart when possible. In this study, we evaluated the expression of several early cardiac stem cell- and proliferation- associated biomarkers in adult human cardiac tissue from the left ventricle (LV) and the potential stem cell niche region the atrioventricular junction (AVj)3,4,5. Specifically, we sought to investigate whether global ischemia, caused by cardiac arrest, activate regenerative processes such as cardiomyocyte remodeling and cell renewal in the adult human heart.
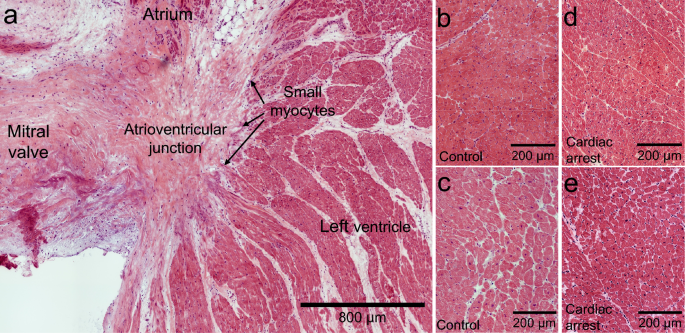
When damage occur in cardiac tissue, the result is often an increase in fibrosis, hypertrophy, adipose tissue infiltration, lipofuscin accumulation or nuclei fragmentation. We expected histological changes in the cardiac arrest group, but no clear difference was observed by the systematic analysis of the tissue. The time window, 2–4 days between the cardiac arrest and organ donation, seems too short for a histological remodeling of the normal myocardium to be demonstrated.
To further investigate potential differences between the two groups, the expression of cTnT, a part of the sarcomeres of cardiomyocytes, was studied. As expected, an even distribution was found in LV from the control group. In contrast, after cardiac arrest individual cardiomyocytes with a decreased expression of cTnT were discovered. Moreover, our results show that these cardiomyocytes with low cTnT upregulated the expression of the stem cell associated biomarkers. This is an interesting similarity to transient cardiomyocyte dedifferentiation, which is characterized by structural remodelling of the sarcomeres including decreased levels of cTnT7,9. We have previously reported that MDR1, SSEA4 and WT1 are expressed in small immature myocytes in the suggested hypoxic stem cell niche in the human AVj but not in LV from the same hearts 4. Upregulation of these early cardiac biomarkers in a subpopulation of LV cardiomyocytes post ischemia supports the idea of dedifferentiation in existing cardiomyocytes. Furthermore, a study using brief non-lethal myocardial ischemia–reperfusion model in sheep reported that MDR1 was upregulated both after 3 and 48 h following reperfusion23. The authors proposed MDR1 as an early biomarker whose activation plays a pivotal role for cell survival. The expression of MDR1 in human LV following reperfusion after cardiac arrest in our study is consistent with a prolonged expression in response to global ischemia.
In addition, we found expression of the early cardiac transcription factor NKX2.5 in the LV cardiomyocytes with reduced cTnT interpreted as a potential reprogramming of adult cardiomyocytes into a more immature phenotype. NKX2.5 expression was detected in dedifferentiating rat cardiomyocytes in culture9 and is known to lie upstream of many essential genes for heart development13. Furthermore, in zebrafish, it was shown that activation of NKX2.5 was required for not only adult myocardial repair but also to provoke the associated proteolytic pathways of sarcomere disassembly as well as the proliferative response for cardiomyocyte renewal24. In line with this background, we suggest that the increase in NKX2.5 in combination with a decrease in cTnT expression may signify a remodeling process. Collectively, a reduction in cTnT and upregulation of stem cell associated biomarkers after an episode of global ischemia followed by few days of oxygen supply indicate a remodeling of the LV cardiomyocytes.
Intriguingly, hypoxia has been shown to induce dedifferentiation of early committed cells into pluripotency25. The fact that no nuclear Hif1α expression was observed in the LV after cardiac arrest despite the increased MDR1 expression after 1–4 days of reperfusion is likely due to the very short half-life (~ 5–8 min) of Hif1α after return to normal oxygen levels26,27. Under normal conditions Hif1α is expressed in the cytoplasm18, but when the oxygen levels drop Hif1α is instead accumulated in the nuclei17. Beyond its function as a transcriptional regulator for the cellular response to hypoxia, Hif1α plays a role in the activation of genes related to tissue repair28. In contrast to the findings in LV, nuclear Hif1α expression was detected in non-cardiomyocytes in the AVj in both groups, as previously reported4,5. This indicates that the AVj region holds a lower oxygen level than other parts of the heart, strengthening the theory of hypoxic stem cell niche in adult human heart. A hypoxia-responsive element has been identified in the early cardiac transcription factor WT1 sequence that bounds to Hif1α which was required for activation of the WT1 promotor29. WT1 has been correlated to epicardial regeneration30 as well as expression by endothelial cells31. We have previously reported that WT1 is expressed in the human AVj but not in LV cardiomyocytes4. In the present study, the co-expression of WT1 was found in small SSEA4+/cTnT+ myocytes in AVj. The numbers of WT1+/cTnT+ cells increased in the AVj after cardiac arrest, interpreted as a regenerative response to global hypoxia in the niche region. Another observation was increased numbers of WT1+/cTnT− cells in LV after cardiac arrest (data not shown) interpreted as an activation of non-myocytes.
It is common for cardiomyocytes to have more than one nucleus. The nuclei are separated from each other in cardiomyocytes. The analyses of PCM1 expression revealed twin nuclei in cardiomyocytes. A systematic quantification of multiple large images showed that the number of twin nuclei increased after cardiac arrest, in both locations. The highest numbers were counted in the LV. Donor 21 was an outlier showing highest number of twin nuclei after the longest period of hypoxia (75 min) compared to the others (Suppl. Table 1). However, it is difficult to draw conclusions from only one case. Binucleation takes place during the fetal development32. The absence of Ki67 or PCNA expression in the twin nuclei in the LV suggest that the results represent binucleation rather that proliferation. However, it should be noted that the half-lives of these two proliferation markers are short (~ 1 and 8 h respectively)20,21. Although we cannot solidly determine whether the twin nuclei represent ongoing cell division or binucleation, it is worth noting that both these processes reflect mitosis33,34. Furthermore, it has been shown that PCM1 is a centrosome protein which localizes to the nuclear membrane2 and more specifically to dense structures on the cytoplasmic site of the nuclear envelope35. Therefore, the appearance of the PCM1 staining in the twin nuclei with two visible nuclear envelopes (see Fig. 5b2,c2,d) is in itself evidence which strongly suggests binucleation rather than polyploidy within a single nucleus. In line with our results, double nuclei were observed in dedifferentiating cardiomyocytes days after apical resection in newborn mice, whereas neighbouring myocytes which did not undergo dedifferentiation or associated sarcomeric disorganization only displayed single nuclei36. Thus, regardless of whether they were destined for cell division or binucleation, the twin nuclei are consistent with a remodeling process.
Neither of the proliferation markers were found in cardiomyocytes in the LV after cardiac arrest, not even in the cardiomyocytes with the low cTnT expression suggesting that remodeling is a longer process, and that proliferation has not been initiated 1–4 days following cardiac arrest. Support for this can be found in the study by Meckert et al. who found 12% of the LV myocytes contained Ki67+ nuclei in 7–13 days-old infarcts. Earlier (1–6 days) and also later (14–21 days), the portion of Ki67+ myocytes was significantly lower37. The absence of Ki67+ nuclei in the LV in the present study (1–4 days after cardiac arrest) therefore seems to be largely in agreement with these results.
In contrast to the LV, PCNA and Ki67 were co-expressed with cardiac specific nuclei marker PCM1 in AVj, which may indicate increased proliferation in small myocytes after a period of global hypoxia. Ki67 has a shorter half-time than PCNA20,21, which could be an explanation to why more of PCNA+/PCM1+ nuclei compared to Ki67+/PCM1+ nuclei were detected. Another possibility is that PCNA can also be involved in DNA repair, including in human cardiomyocytes37. As there were clear examples of PCNA+/PCM1+ as well as Ki67+/PCM1+ twin nuclei in AVj, it appears that at least some of the PCNA positivity was associated with nuclei which had entered the cell cycle. Previously, we reported increased numbers of BrdU+ proliferating cells in the AVj using physical exercise in the adult rats3. In addition we have shown expression of biomarkers related to hypoxia, cardiac stem cells, proliferation and migration in the left and right AVj4,5 indicating that this region is of importance to cardiomyocyte cell renewal in human. I the current study, the increased expression of proliferation markers in the AVj after cardiac arrest suggests that more cardiomyocytes might had entered the cell cycle.
What may be the ultimate fates of the PCM1+ cardiomyocytes in AVj that displayed cell cycle markers? Regarding some of the PCM1+ nuclei that displayed no clear PCM1+ nuclear envelopes (Fig. 4a2,a3), these are admittedly difficult to interpret. However, there is evidence to suggest that the insoluble perinuclear matrix remains in most phases of the cell cycle but disassembles only in pro-metaphase and metaphase of mitosis, making it possible to visualize myocyte nuclei almost throughout the whole cell cycle38. It thus seems possible that some of the Ki67+/PCM1+ and PCNA+/PCM1+ nuclei in the AVj in the present study were in prometaphase and metaphase.
In a study on infarcted human hearts, a low number of Ki67+ myocytes in the periinfarct zone had appearances consistent with conventional mitosis37. Thus, there is a slight possibility that minor portion of the Ki67+/PCM1+ and PCNA+/PCM1+ nuclei in the AVj may represent conventional cell division. However, Meckert et al. reported evidence to suggest that in human infarcts, entrance of cardiomyocytes into the cell cycle is transient and that endomitosis, leading to polyploidy rather than mitosis, is the final fate of cycling cells37. Nevertheless, since cardiac arrest and myocardial infarction are different conditions, there is a clear need for further studies into these issues. A possible explanation behind the differences between the AVj and the LV in the present study may be that the cardiomyocytes in the AVj are younger and in a more immature stage and thus perhaps able to express proliferation markers early after global ischemia.
Some limitations of the present study should be acknowledged. Immunohistochemistry data shows only a snapshot in time but provide important insights on co-expression of biomarkers in human adult cardiomyocytes. The low number of individuals and the limited range of the reperfusion period after cardiac arrest, as well as the short half-life of the chosen proliferation markers, makes it challenging to ascertain whether the twin nuclei were destined for binucleation, polyploidization or cell division. Also, some of the Ki67 and PCNA positivity may have been reflective of polyploidization and/or DNA damage, both of which may have occurred to varying extents. The methods and markers that we used did not allow us to investigate whether this was indeed the case. The physiological significance of the increased number of twin-nuclei as well as the Ki67+/PCM1+ and PCNA+/PCM1+ nuclei in and the remodelling cardiomyocytes after cardiac arrest thus needs further investigation. Nevertheless, the material is highly unique and may provide important insights into cellular response to cardiac arrest in human heart and clues for therapies aimed at improving heart regeneration.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-024-65212-z
