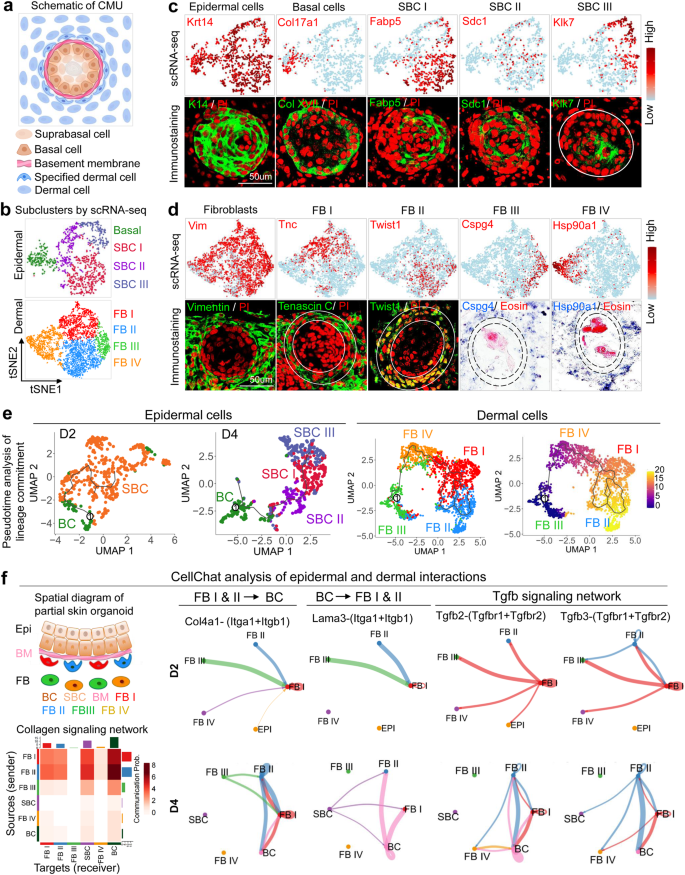
Transcriptome profiling during the formation of epidermal–dermal spheroids
When grown as an explant culture, combined dissociated newborn mouse epidermal and dermal cells form a spheroid composed of 1–3 layers of dermal cells on the outside overlying an epidermal cell ring encircling keratin debris within the center (Fig. 1a). The spheroids either form a planarized skin or maintain a spheroid topology after transplantation onto the dorsum of nude mice (Supplementary Fig. 1a). K14 immunostaining shows that hair follicles can form either from the spheroids (15 ± 5%) or the later staged planarized skin (85 ± 5%) (Supplementary Fig. 1a). We previously demonstrated that the cysts coalesce to form a planarized skin in newborn mouse cell culture, whereas adult mouse cells grown in explant cultures form epidermal aggregates but do not form these epidermal-dermal coupled spheroids or planarized skin nor hair follicles14. Therefore, this finding suggests that the skin spheroid represents a unique morphological marker for morphogenesis in cultured reconstituted skin explants. By quantifying the cellular components at the middle cross-section level, we observe that there are 15–35 basal epidermal cells, 3–5 layers of suprabasal epidermal cells, a basement membrane, and 1–4 layers of dermal cells.
a Schematic drawing of a CMU. b Cell clustering of newborn mouse epidermal and dermal cells of combined D2 & D4 cultures. c TSNE plots and immunostaining for expression of exemplary genes in newborn mouse epidermal cells, basal cells, suprabasal cells I (SBC I), SBC II, and SBC III. d TSNE plots and immunostaining for expression of exemplary genes in newborn mouse dermal fibroblasts (FB), FB I, FB II, FB III, and FB IV. e Pseudotime analysis of lineage commitment of newborn mouse epidermal (D2 and D4) and dermal cells (D4). f CellChat analysis of newborn mouse epidermal and dermal interactions during CMU formation. False discovery rate < 0.05 and Log2 fold change > 1.
To examine the cellular and molecular components of these spheroids, we profiled 8558 single cells from D2 (day 2) and D4 (day 4) newborn mouse skin explant cultures using scRNA-seq (Supplementary Fig. 1b and Supplementary Table 1). T-distributed stochastic neighbor embedding (tSNE) plots of D2 and D4 newborn mouse skin explant cultures reveal 11 cell clusters, with epidermal cells and dermal fibroblasts as the two major sub-clusters (Fig. 1b). Since polarization occurs in epidermal cells (K14+), we isolated these cells and performed tSNE plot analysis, which reveals four main epidermal cell clusters (Fig. 1c) including basal cells (Col17a1+) and suprabasal cells I (SBC I, Fabp5+), SBC II (Sdc1+), and SBC III (Klk7+) (Fig. 1c and Supplementary Fig. 1c). ScRNA-seq analysis also reveals four dermal fibroblast groups including fibroblast I (FB I)–FB IV, which show high expression of representative genes such as Tnc, Twist1, Son, and Hsp90a1, respectively (Fig. 1d), as well as other highly expressed genes (Supplementary Fig. 1c). Pseudotime analysis by Uniform Manifold Approximation and Projection (UMAP) plots reveals that the basal layer can differentiate into the suprabasal layer at D2, which can further differentiate into another two suprabasal cell types at D4 (Fig. 1e). Pseudotime analysis also reveals that FB III can undergo lineage commitment to FB IV, FB I to FB II, with specific molecular identity in each cell cluster (Fig. 1e and Supplementary Fig. 1c).
Epidermal–dermal coupling is required for the establishment of competent morphogenesis units
To examine whether epidermal cells interact with dermal cells to form functional CMUs, we isolated epidermal and dermal cells from the D2 and D4 newborn mouse skin explant cultures, in which six main clusters were characterized by tSNE plots, including four dermal fibroblast clusters (FBs I–IV) and two main epidermal clusters (BC & SBC), (Fig. 1f and Supplementary Table 2). We then used CellChat to study the communications between epidermal and dermal cells21. CellChat analysis of these six clusters identified FB I, FB II, and basal cells as the dominant communication “hubs” that secrete and receive signals (Supplementary Fig. 1d). FB I and FB II secrete the majority of Collagen whereas basal cells highly express Laminin. Collagen and Laminin are the top 2 secreted ECM outgoing signaling patterns (Supplementary Fig. 1d left). Basal epidermal cells can receive the vast majority of ECM signaling secreted by both dermal cells and epidermal cells (Supplementary Fig. 1d right). This implies that molecular interactions occur between epidermal and dermal cells.
The CellChat-inferred Collagen signaling pathway further confirms that FB I and FB II are the primary ligand sources, which act not only in an autocrine manner between dermal cells but can act even more strongly as paracrine signals from dermal to epidermal cells (Fig. 1f lower left). Indeed, CellChat identified ligand-receptor pairs including Collagen-Integrin as the most significant signaling contributors from FB I and FB II to basal cells (Fig. 1f). FB I- and FB II-expressed Collagen IV can be received by Integrins a1 and b1 in basal cells. Conversely, CellChat identified Laminin-Integrin as the major signaling molecule for communication from basal cells to FB I and FB II. Here, basal cells express Laminin signals that can be transmitted to FB I and II which also express Integrins a1 and b1. These laminin-integrin interactions might induce CMU formation. Interestingly, epithelial–mesenchymal interactions (EMI) are enhanced during the CMU formation process from D2 to D4, as indicated by the increased interactions of Collagen and Laminin at D4 (Supplementary Fig. 1e). CellChat analysis also shows that FB I and II secrete most of the signaling molecules, which can be received by Basal cells (Supplementary Fig. 1f). For example, Tgfb signaling pathway genes are highly expressed in FB I and FB II and can be received by Tgfbr1 and Tgfbr2 expressed by the basal cells (Fig. 1f right). Together, these data suggest that dermal cell-derived ECM signaling may regulate epidermal cell behavior, and vice versa during CMU formation.
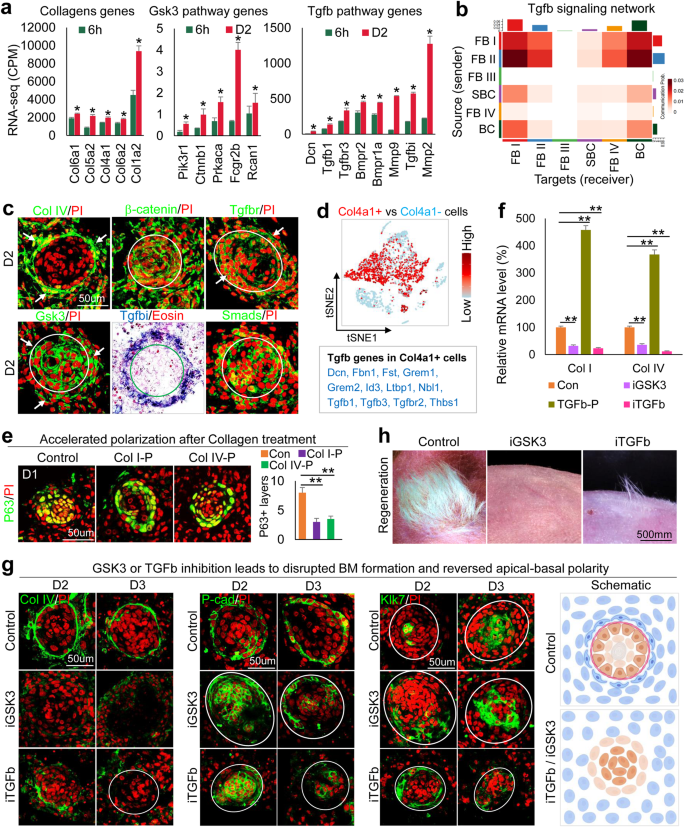
Tgfb and Gsk3 regulate ECM expression and basement membrane organization to control epidermal apical-basal polarity
Since the CMU is composed of three cellular components, we began to explore how the basement membrane formation influences epidermal apical–basal polarity. RNA-seq analysis of D2 (polarized state) and 6h (unpolarized state) newborn mouse skin explant samples revealed that the ECM gene ontology (GO) terms rank prominently (Supplementary Fig. 2a). ECM genes (e.g., Collagens) are significantly increased from 6h to D2 (Fig. 2a, Supplementary Fig. 2b, and Supplementary Table 2). Interestingly, we also observed that Gsk3 pathway-related genes are significantly increased from 6h to D2 by bulk RNA-seq analysis (Fig. 2a and Supplementary Fig. 2c). TSNE plots show that Gsk3b colocalizes with Col1a1 and Col4a1 in dermal cells but not in epidermal cells (Supplementary Fig. 2d), which is verified by immunostaining (Fig. 2c). Since Gsk3 is a Wnt signaling inhibitor, we checked b-catenin expression in D2 cultures and observed that it is not activated in specified dermal cells (Fig. 2c), indicating Gsk3 may function to repress Wnt signaling. To test the involvement of GSK3+ on collagen expression, we isolated Gsk3+ dermal cells and performed GO analyses, which revealed several ECM molecule terms including collagen that may act downstream of Gsk3 (Supplementary Fig. 2e).
a RNA-seq analysis shows that ECM, Gsk3, and Tgfb pathway genes are upregulated from 6h to D2. *p < 0.05, n = 2. b Heatmap of senders and receivers of Tgfb signaling. c Immunostaining and in situ hybridization show gene expression in D2 culture. d Tgfb pathway genes are highly expressed in Col4a1+ vs. Col4a1- cells. e Immunostaining and quantification for P63 show accelerated polarization at D1 after the addition of Collagens I or IV at D0. **p < 0.01. N ≥ 3. f qRT-PCR analysis reveals Col1a1 and Col4a1 expression after inhibition of GSK3 (iGSK3) or iTGFb or the addition of TGFb recombinant protein (TGFb-P). **p < 0.01. N = 3. g Immunostaining of Collagen I, P-cadherin, and Klk7 expression after GSK3 or TGFb inhibition. h Hair regeneration with transplantation of iGSK3- or iTGFb-treated cultures.
To probe the upstream regulators of Gsk3 that modulate ECM expression, we analyzed our bulk RNA-seq data which reveals that Tgfb pathway genes are significantly increased from 6h to D2 in newborn mouse skin explant cultures (Fig. 2a). Using CellChat secreted signaling mode analysis, we observed that Tgfb signaling pathway genes are highly expressed in FB I and FB II (Fig. 2b), with multiple Tgfb ligand-receptor pairs contributing to the EMIs (Supplementary Fig. 2f). In situ hybridization and immunostaining showed that Tgfb pathway genes are expressed in the specified dermal cells and the basal epidermal cells (Fig. 2c). Since Col4a1 is highly expressed in FB I and FB II, we analyzed the gene expression profile in Col4a1+ cells versus Col4a1- cells and observed that Tgfb pathway genes are indeed highly enriched in Col4a1+ cells (Fig. 2d). We then evaluated the properties of the FB II cell cluster which is the biggest source of Tgfb signaling (Fig. 2b). Four sub-types of fibroblast cells (FB II-A, B, C, D) can be further identified when the FB II cluster was subjected to a deeper tSNE plot analysis. FB II-A shows high expression of dermal papilla marker genes including Bmp4, Bmp7, Lef1, and Ltbp1 (Supplementary Fig. 2g). These data suggest that the Tgfb+ FB II-A cell population contains the cells that are potentially the competent cells for prospective regeneration (e.g., the pre-dermal condensate cells) during CMU formation.
Since Collagens I and IV are known basement membrane components, we tested their function by adding Collagen I or IV recombinant protein to the skin explant cultures. Exogeneous Collagen proteins can be incorporated into the basement membrane region and dermal cells. This leads to accelerated basement membrane formation and mature polymerized collagen fibers in the collagen-treated groups compared to the control group which shows immature collagen expression and a relatively incomplete basement membrane (Supplementary Fig. 2h). The addition of Collagen I or IV did not significantly change the aggregate size, but dramatically increased the dermal cell layers surrounding the aggregate (Supplementary Fig. 2i). As a result, the polarization process is accelerated in the Collagen-treated groups compared to the control at D1, as shown by P63 immunostaining (Fig. 2e). This suggests that dermal cell-secreted Collagens promote epidermal cell polarization in newborn mouse skin explant culture.
Since the expression of Gsk3 pathway genes is increased from 6h to D2 and Gsk3 colocalizes with Col1a1 and Col4a1 in dermal cells (Fig. 2a and Supplementary Fig. 2c and d), we tested if Gsk3 signaling regulates Collagen expression by treating D0 cultures with a Gsk3 inhibitor, Chir99021. We observed that Col1a1 and Col4a1 mRNA and protein levels are significantly decreased at D2 compared to controls as shown by qRT-PCR and immunostaining, respectively (Fig. 2f, g and Supplementary Fig. 2k). Particularly, while GSK3 inhibition does not influence aggregate size (Supplementary Fig. 2i), it decreased Collagen I and IV expression in the specified dermal cells (Fig. 2g left and Supplementary Fig. 2k). This resulted in incomplete basement membrane formation (Fig. 2g) and decreased b1-integrin expression in the basal epidermal aggregate compared to the control (Supplementary Fig. 2m). Without the dermal ECM and basement membrane, the apical-basal polarity is perturbed or even reversed when GSK3 is inhibited, as revealed by P-cadherin and Klk7 immunostaining (Fig. 2g middle and right). These results indicate that GSK3 regulates ECM expression and basement membrane formation to modulate the epidermal cell apical-basal polarity. Accordingly, abrogation of apical-basal polarity by inhibition of GSK3 in newborn mouse skin explant cultures results in significantly decreased hair regeneration compared to the control (Fig. 2h).
Because Tgfb expression is also increased from 6h to D2 and colocalizes with Gsk3 and Collagens (Fig. 2a–c), we treated the D0 newborn mouse skin explant cultures with Tgfb recombinant protein which led to significantly increased mRNA expression of Gsk3a, Gsk3b, Col1a1, and Col4a1 at D2 (Supplementary Fig. 2j). While inhibition of Tgfb activity at D0 led to dramatically decreased mRNA expression of these genes at D2 (Fig. 2f and Supplementary Fig. 2j), suggesting that Tgfb regulates Gsk3 and ECM expression. Inhibition of Tgfb resulted in significantly lower Collagen I and IV expression in the specified dermal cells and impaired basement membrane formation compared to the controls (Fig. 2g and Supplementary Fig. 2k), smaller epidermal aggregates, and fewer dermal cell attachments (Supplementary Fig. 2i).
Inhibiting the Tgfb pathway also results in dramatically decreased b1-integrin expression in the outer epidermal aggregate layer at D2 and D3 in newborn mouse skin explant cultures (Supplementary Fig. 2m), suggesting a decreased EMI. Immunostaining showed that E-cadherin is not polarized in the outer layer of the Tgfb inhibition group compared to the control (Supplementary Fig. 2n). Intriguingly, P-cadherin has a higher expression in the inner layers but Klk7 has a higher expression in the outer layers of the epidermal aggregate in the Tgfb inhibition group (Fig. 2g and Supplementary Fig. 2n), indicating the apical-basal polarity has been inverted. This confirms that Tgfb regulates CMU formation (Fig. 2g schematic). Likewise, transplantation of Tgfb-inhibited cells to the back of nude mice results in significantly fewer regenerated hairs compared to the control (Fig. 2h). Together, this provides further compelling evidence that CMU formation in reconstituted newborn mouse skin explant morphogenesis influences the outcome of hair regeneration upon transplantation.
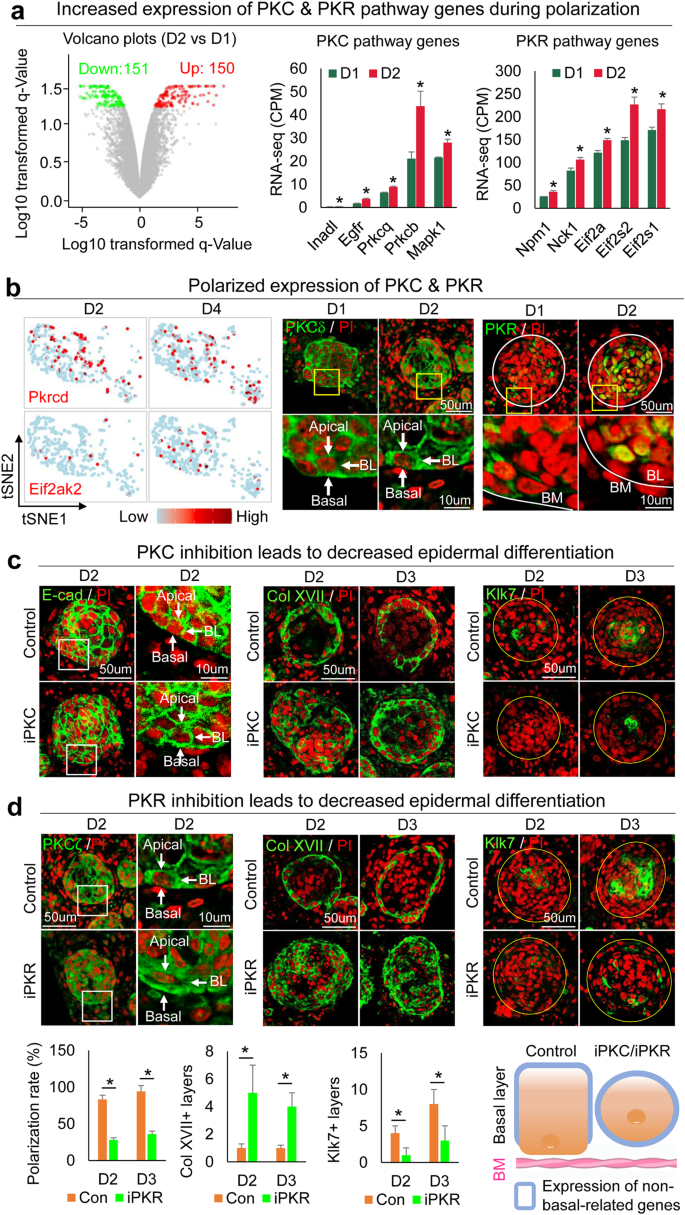
PKR–PKC axis promotes apical-basal polarity formation in epidermal cells positioned in the outer layer of cell aggregates
Since polarization occurs in the epidermal aggregate, we then studied how epidermal cell polarization influences CMU formation. Gene ontology analysis of D2 upregulated genes in newborn mouse skin explant cultures showed that highly enriched genes are involved in keratinization, keratinocyte differentiation, epidermal cell differentiation, etc. (Fig. 3a, Supplementary Fig. 3a-b, and Supplementary Table 3), which mainly include PKC and PKR pathway genes that may regulate epidermal differentiation (Fig. 3a and Supplementary Fig. 3c). Representative PKR and PKC pathway genes are expressed in both basal and suprabasal cells, and the number of expressing cells is increased from D2 to D4 in newborn mouse skin explant cultures as seen in tSNE plots (Fig. 3b and Supplementary Fig. 3d). Immunostaining confirms that PKCa and PKCδ are expressed in both basal and suprabasal cells. D2 epidermal aggregate basal cells show polarized expression (Fig. 3b and Supplementary Fig. 3d). PKR expression is increased from D1 to D2, particularly in the suprabasal cells (Fig. 3b).
a RNA-seq analysis shows PKC and PKR pathway genes upregulated from D1 to D2. *p < 0.05, n = 2. b TSNE plots and immunostaining of representative PKC (Pkrcd) and PKR (Eif2ak2) pathway gene expression in skin organoid cultures. c Immunostaining of E-cadherin, Collagen XVII, and Klk7 shows inhibition of PKC (iPKC) leads to decreased polarization at D2 and D3. d Immunostaining and quantification of PKCζ, Collagen XVII, and Klk7 show inhibition of PKC (iPKC) leads to decreased polarization at D2 and D3. *p < 0.05. N ≥ 3.
To test if PKC regulates epidermal apical–basal polarity, we inhibited PKC function by adding a specific inhibitor, Bisindolylmaleimide I, to the culture at D1 newborn mouse skin explant cultures. K14 immunostaining shows that epidermal cell aggregation is not influenced (Supplementary Fig. 3f), but the polarized expression of E-cadherin in the basal layer is abolished by this treatment at D2 and D3 (Fig. 3c and Supplementary Fig. 3f). Collagen XVII and P-cadherin are increased but Klk7 is decreased in the epidermal cyst in the PKC inhibition group compared to that of the control group at D2 and D3 (Fig. 3c and Supplementary Fig. 3f). To examine if PKR regulates PKC at the mRNA level, we inhibited PKR function with imidazolo-oxindole and found that Prkca expression did not change significantly (Supplementary Fig. 3e). However, inhibiting PKR blocked PKC polarization (Fig. 3d left), indicating that PKR regulates PKC at the protein level. Furthermore, inhibiting PKR led to increased Collagen XVII and decreased Klk7 expression (Fig. 3d), and abolished polarization of E-cadherin and P-cadherin in the basal layer of the epidermal aggregate (Fig. 3d and Supplementary Fig. 3f). These findings indicate that PKR regulates PKC polarization which contributes to apical-basal polarity (Fig. 3d and Supplementary Fig. 3g). To confirm this, we added both PKR and PKC inhibitors to the D0 culture and observed decreased epidermal differentiation, without obvious influence of cell apoptosis at D2 and D3 newborn mouse skin explant cultures (Supplementary Fig. 3h-i).
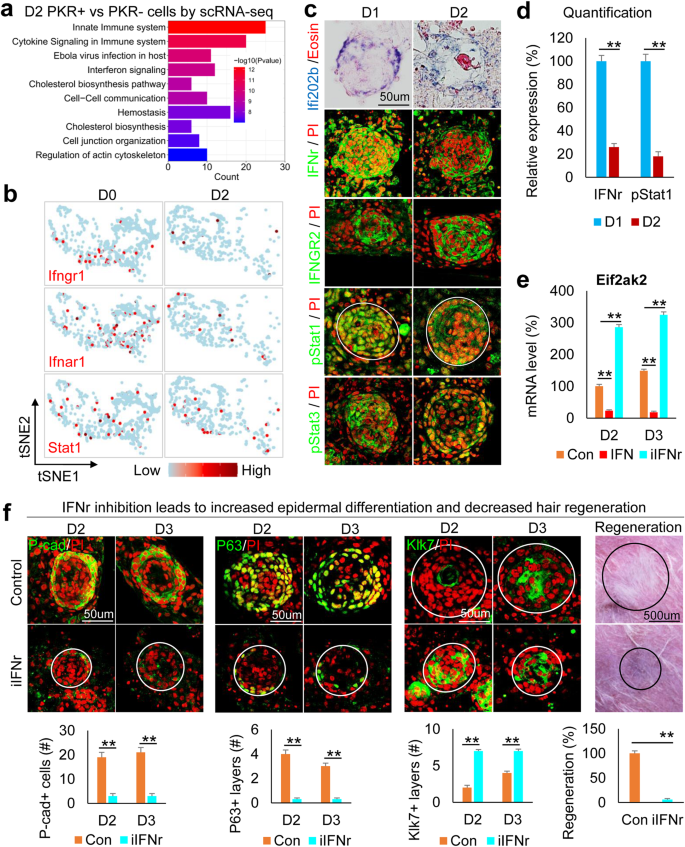
IFNr suppresses PKR–PKC axis in the epidermal basal cells to maintain their multipotency
We next sought to identify upstream regulators of the PKR–PKC axis that control apical-basal polarity formation. We compared gene expression in PKR+ vs. PKR–epidermal cells by analyzing the D2 (polarized stage) scRNA-seq data of newborn mouse skin explant cultures. The top four GO terms are all related to interferon pathways in PKR+ vs. PKR-cells (Fig. 4a and Supplementary Table 4). Surprisingly, tSNE plots revealed that the number of cells expressing key interferon pathway genes (e.g., Ifngr1, Ifnar1, and Stat1) decreased from D0 to D2 (Fig. 4b). Immunostaining and in situ hybridization showed that Ifi202b, IFNr, IFNGR2, pSTAT1, and pSTAT3 are decreased from D1 to D2 newborn mouse skin explant cultures, implying that the interferon pathway may negatively regulate the polarization process (Fig. 4c, d). Adding IFNr recombinant protein suppresses Eif2ak2 (encodes PKR) whereas inhibiting IFNr enhances Eif2ak2 expression (Fig. 4e). These results suggest IFNr negatively regulates Eif2ak2 expression. Inhibiting IFNr led to dramatically smaller aggregate formation compared to the control (Supplementary Fig. 4a). The expression of P-cadherin, P63, Collagen XVII, and E-cadherin is significantly decreased and the expression of Klk7 is significantly increased in the IFNr-treated group compared to the control group (Fig. 4f and Supplementary Fig. 4b, c), indicating that the IFNr is required to maintain the multipotency of the CMU. Perturbed CMU formation in the IFNr-treated cultures led to significantly decreased hair regeneration upon transplantation compared to the control (Fig. 4f).
a Ontology of gene expression in PKR+ vs. PKR− cells in D2 culture. b TSNE plots show representative interferon pathway genes in epidermal cells. c Immunostaining and in situ hybridization for representative interferon pathway gene expression in D1 and D2 cultures. d Quantification of pStat1 expression. **p < 0.01. N ≥ 3. e Eif2ak2 expression after addition or inhibition of IFNr. **p < 0.01. N = 3. f Immunostaining and quantification show that inhibition of IFNr results in decreased P-cadherin and P63 but increased Klk7 expression at D2 and D3. Hair regeneration is significantly decreased after transplantation of iIFN-treated cells compared to the control. **p < 0.01. N = 5.
Interestingly, we also observed that inhibiting IFNr led to disrupted basement membrane (Laminin+) formation (Supplementary Fig. 4d) and decreased Collagen I assembly surrounding the epidermal aggregate (Supplementary Fig. 4e), implying that epidermal cells can interact with the specified dermal cells. In addition, without the expression of ECM in the specified dermal cells in the IFNr-treated group, the ECM receptor b1-integrin is dramatically decreased in the basal layer of the epidermal aggregate (Supplementary Fig. 4f). These results further confirm the epidermal-dermal communications suggested by CellChat analyses.
VEGF mediates dermal cell attachment to the epidermal cyst
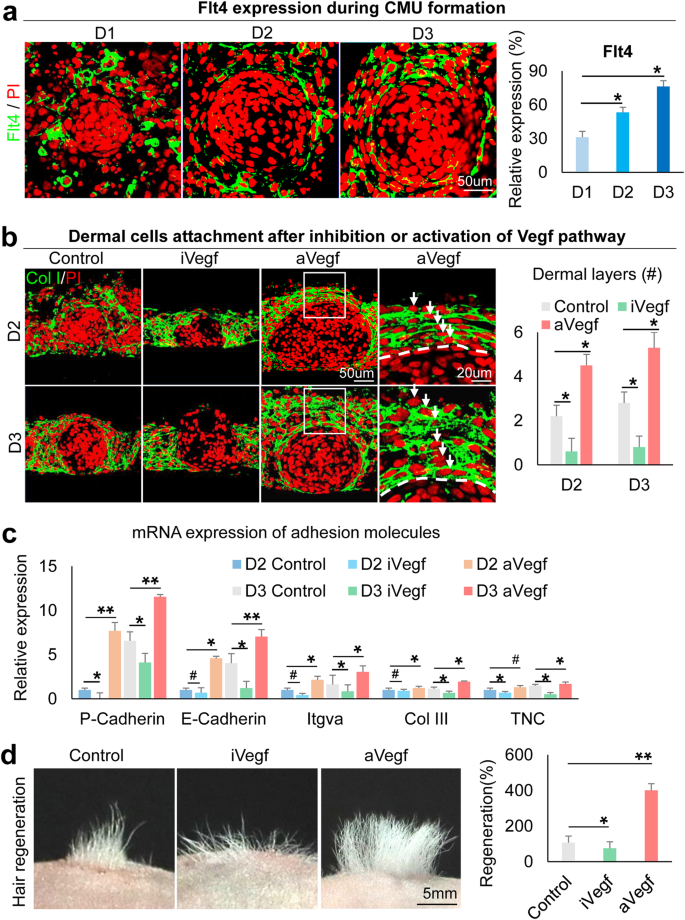
Our previous study confirmed that dermal cells are required for epidermal cell self-organization14. RNA-seq analysis showed VEGF signaling pathway genes are significantly increased at D2 vs. 6h newborn mouse skin explant cultures (Supplementary Fig. 5a). We examined the expression of Flt4, which is significantly upregulated among VEGF signaling pathway receptors (Supplementary Fig. 5a). Immunostaining shows that Flt4 is gradually increased in the specified dermal cells from D1 to D3 in newborn mouse skin explant cultures (Fig. 5a). We then treated the cultures with Axitinib (an inhibitor of VEGF receptors) or VEGF recombinant protein to evaluate the function of VEGF signaling. Intriguingly, we observed that the addition of VEGF recombinant protein results in significantly more dermal cell layers attached to the epidermal aggregate compared to the control, and vice versa in the VEGF inhibition groups. Immunostaining for K14, Collagen IV, and Laminin shows that VEGF inhibition leads to the formation of a smaller aggregate and an abolished basement membrane compared to the control (Fig. 5b and Supplementary Fig. 5b, c). qRT-PCR reveals that adhesion molecules expressed in both epidermal cells (e.g., P-cadherin, E-cadherin, and Itgva) and dermal cells (e.g., Collagen III and Tnc) are significantly increased in D2 and D3 after addition of VEGF recombinant protein (Fig. 5c). These data indicate that VEGF signaling regulates dermal cell attachment during CMU formation (Supplementary Fig. 5d). We also validated that exogenous inhibition or addition of VEGF leads to decreased or increased Flt4 expression in specified dermal cells, respectively (Supplementary Fig. 5e). Transplantation of VEGF recombinant protein-treated cultures into nude mice results in significantly more hair formation compared to the control, and vice versa in the VEGF inhibition group (Fig. 5d). This further suggests that proper dermal cell attachment is required for hair regeneration.
a Immunostaining shows Flt4 expression is gradually increased in dermal cells surrounding the epidermal aggregate at D1–D3. *p < 0.05. N ≥ 3. b Immunostaining for Collagen I shows that inhibition of VEGF results in decreased dermal cell attachment and vice versa at D2 and D3. *p < 0.05. N ≥ 3. c qRT-PCR shows cell adhesion expression after VEGF inhibition or activation. *p < 0.05. #, no statistical significance. N = 3. d Hair regeneration with transplantation of iVegf or aVegf-treated cells. I, inhibition; a, activation. *p < 0.05 and **p < 0.01. N = 4.
Restoring the hair regeneration ability of adult mouse cells by re-establishing morphogenetic competence
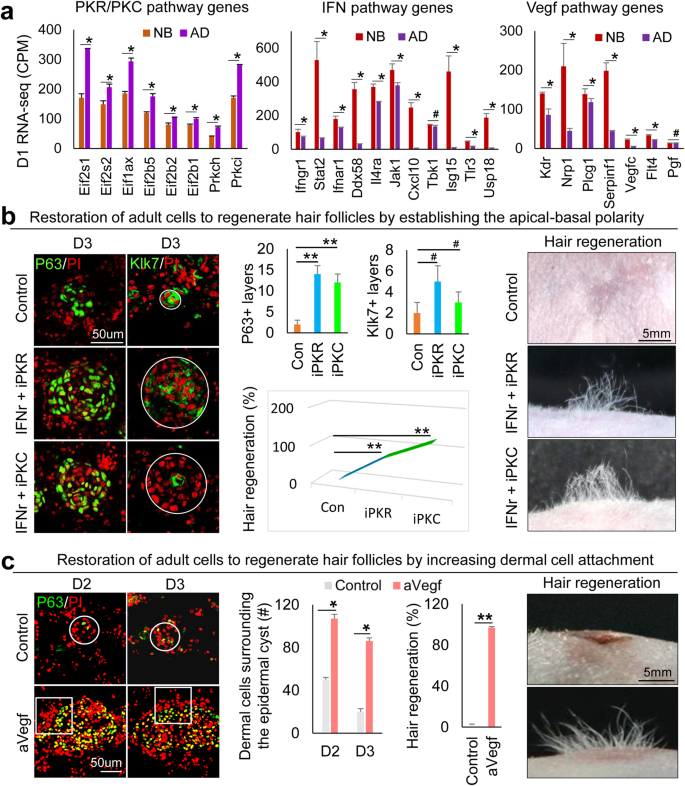
Adult mouse cells lose hair regenerative abilities after culture13,14. This may be due to excessive epidermal differentiation and weak EMI that leads to failed CMU formation. Using RNA-seq analysis, we observed that PKC and PKR pathway genes are significantly increased, but IFN and VEGF pathway genes are significantly decreased in D1 adults compared to newborn (unpolarized stage) mouse cell cultures (Fig. 6a). Immunostaining confirmed the increased expression of PKC and PKR, and decreased expression of IFNr and Flt4 in adult mouse cells compared to newborn mouse cells (Supplementary Fig. 6a). Using a modified environmental reprogramming protocol (Supplementary Fig. 6b), we next tried to reactivate the adult mouse cells to form CMU by modulating these molecules. We observed that PKR or PKC inhibition results in larger aggregate formation without the addition of growth factors in adult mouse cell cultures. However, these conditions do not achieve the levels seen in newborn mouse cell cultures (Supplementary Fig. 6c). With IFNr addition and PKR/PKC inhibition, the skin cysts show a polarized structure including basal layers (P63+ and Collagen XVII+) and suprabasal layers (Klk7+), compared to the control groups without apical–basal polarity formation (Fig. 6b and Supplementary Fig. 6d). The enlarged skin cyst in IFNr + iPKR (or iPKC) cultures is not due to cell proliferation (Ki67+) but due to increased cell adhesion (E-cadherin + ) (Supplementary Fig. 6d). A more complete basement membrane (Laminin+) is formed in the restored group compared to the control (Supplementary Fig. 6d). Transplantation of the restored adult mouse skin explant cultures to nude mice results in significantly more hair regeneration compared to the control (Fig. 6b). Using a similar strategy, we successfully restored basement membrane formation and dermal cell attachment by adding VEGF recombinant protein to the adult cell culture (Fig. 6c and Supplementary Fig. 6e, f). This results in significantly more hair regeneration compared to the control (Fig. 6c). These data suggest that the reestablishment of CMU in the mouse skin explant culture is sufficient to restore the regenerative ability of adult cells to form hair follicles upon transplantation.
a RNA-seq comparison of PKR, PKC, IFN, and VEGF pathway genes between newborn and adult at D2. *p < 0.05. #, no statistical significance. N = 2. b Immunostaining and quantification of P63 and Klk7 expression show that inhibition of PKR or PKC results in decreased epidermal cell differentiation in adult mouse cell cultures at D2 and D3. Increased hair regeneration with transplantation of IFNr+iPKR- or IFNr+iPKC-treated adult mouse skin cells. **p < 0.01. #, no statistical significance. N = 8. c Increased dermal cell attachment and hair regeneration after the addition of VEGF recombinant protein (aVEGF) compared to the controls. *p < 0.05, **p < 0.01, # no significant difference. N = 4.
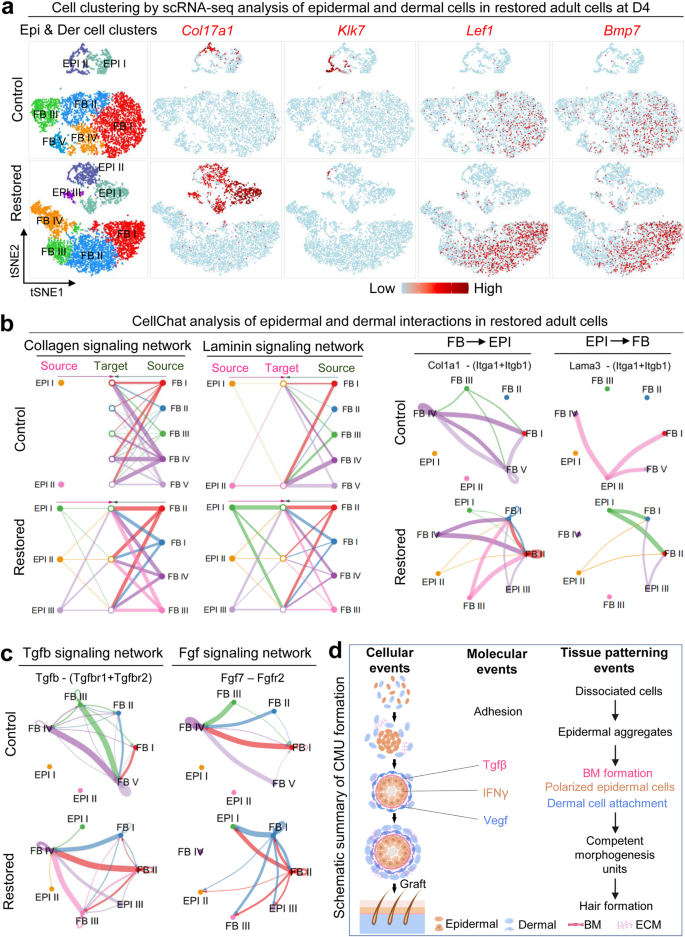
In addition to examining the morphological characteristics of CMU formation, we also checked the molecular identities of the restored adult mouse explant cultures by performing scRNA-seq analysis. Since CMU formation mainly involves EMI, we isolated adult mouse epidermal and dermal cells which were distributed into different sub-clusters (Fig. 7a, Supplementary Fig. 7a, and Supplementary Table 5). TSNE plots reveal that the restored adult mouse skin explants have more basal cells (Col17a1+) and fewer suprabasal cells (Klk7+) than the control group, implying successful maintenance of epidermal multipotency. The number of Lef1+ and Bmp7+ dermal cells (which may constitute the prospective dermal condensate) is also increased in the restored adult mouse skin explants (Fig. 7a). CellChat analysis reveals an increased number of interactions and enhanced weights/strength in restored adult mouse skin explants compared to the control (Supplementary Fig. 7b and Supplementary Table 6), indicating intensive EMIs after environmental reprogramming. More Collagen and Laminin signaling networks are formed between epidermal and dermal cells in the restored adult mouse skin explants (Fig. 7b). For example, dermal cell-secreted Col1a1 signaling can be sensed by Itga1 and Itgb1 expressed by the epidermal cells in the restored group but not in the control group. The epidermal cell-expressed Lama3 can be received by FB I and FB II in the restored group but less in the control group.
a ScRNA-seq analysis shows an increased number of basal cells (Col17a1+), DP cells (Lef1+, Bmp7+), and a decreased number of suprabasal cells (Klk7+) in restored adult cells. b ECM mode of CellChat analysis shows increased EMI in restored adult cells. False discovery rate <0.05 and Log2 fold change >1. c Secreted mode of CellChat analysis shows enhanced Tgfb and Fgf signaling network between epidermal and dermal cells in the restored group. d Summary diagram shows the mechanism of CMU formation.
CellChat secreted mode analysis reveals stronger interactions of ligands and receptors between epidermal and dermal cells in restored adult mouse skin cells compared to the control (Supplementary Fig. 7c). For example, the Tgfb signaling secreted by dermal fibroblasts can be sensed by Tgfbr1 and Tgfbr2 expressed in the epidermal cells in the restored group but not in the control group (Fig. 7c and Supplementary Fig. 7d). FGF signaling which is required for hair regeneration can be secreted by dermal cells and received by epidermal cells in the restored group but not in the control group (Fig. 7c). These data suggest that the EMI is dramatically enhanced at the molecular level after environmental reprogramming. Indeed, scRNA-seq analysis and immunostaining showed an increased number of cells that express dermal papilla markers including Wif1a, Lef1, Sox2, and Rspo3 in the restored group compared to the control (Supplementary Fig. 7e, f). The restored adult mouse skin explants more closely resemble that of the newborn mouse cell cultures, facilitating CMU formation and hair regeneration upon transplantation.
Enhancing the hair regeneration ability of human cells with what we learned from the mouse cells
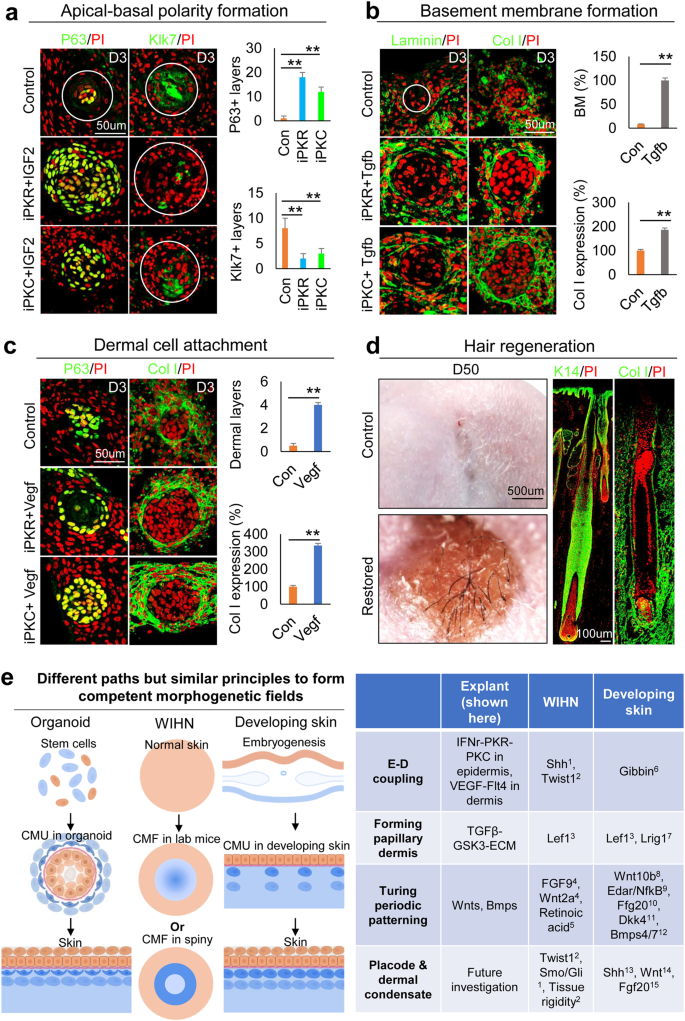
To further test if the principles of CMU formation learned from newborn mouse cell self-organization can be applied to restore human cells’ ability to regenerate hair follicles from skin explants, we modified the culture conditions (Supplementary Fig. 6b) to induce CMU formation from human fetal scalp cells. P63 and Klk7 immunostaining showed that the apical-basal polarity can be established by adding PKR or PKC inhibitors to the cultures of 24-week fetal scalp cells (Fig. 8a). These human epidermal aggregates have enlarged size, stronger cell adhesion, and increased stemness, as indicated by representative markers (Supplementary Fig. 8). Moreover, the addition of TGFβ recombinant protein induce basement membrane formation and increased Collagen I expression (Fig. 8b). Addition of VEGF significantly increased dermal cell attachment surrounding the epidermal cyst compared to the control group (Fig. 8c). Transplantation of these human skin explants to the back of nude mice resulted in prominent hair regeneration, compared to the control which does not have visible hairs (Fig. 8d). Antibodies against human K14 and Collagen I confirmed the neogenic hair follicles are derived from donor cells (Fig. 8d). These data further suggest that the formation of similar epidermal-dermal spheroids in human skin explant culture can enhance hair regeneration upon transplantation onto the nude mice back skin.
Epidermal and dermal cells were harvested from the 24-week human fetal scalp with the same culture condition as the mouse skin explant culture. a Immunostaining and quantification of P63 and Klk7 expression show that inhibition of PKR or PKC results in decreased epidermal cell differentiation in human fetal scalp cell cultures at D3 compared to the controls. **p < 0.01. N = 8. b Immunostaining and quantification of Laminin and Collagen I expression show that addition of Tgfb led to a more complete basement membrane formation compared to the controls. **p < 0.01. N = 8. c Increased dermal cell attachment after the addition of VEGF recombinant protein compared to the controls. **p < 0.01. N = 8. d Hair regeneration after environmental reprogramming. Immunostaining for K14 and Collagen I which only stain human cells in the reconstituted skin. N = 4. e A comparing skin explant, wound-induced hair regeneration (WIHN), and normal developing skin shows that all of them have to form the competent morphogenetic field (CMF) before Turing patterning can take place. They may show different molecular paths but similar principles were used. See references in supplementary information.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41536-023-00340-0