Ethics statement for human samples and animal models
Written informed consent was obtained from healthy donors, and discarded human umbilical cord tissues were acquired according to the approved protocol of the Institutional Review Board (IRB) at the Affiliated Hospital of Jiangsu University. And conducted in accordance with ethical principles of the World Medical Association Declaration of Helsinki. All animal experimental protocols were approved by the Animal Care and Use Committee of Jiangsu University (Approval number: 2020280). All animal experiments were performed in accordance with the associated relevant guidelines and regulations for working with live vertebrate animals.
Animals
Sprague-Dawley rat (male, 6–8 weeks old, 180–200 g) were purchased from the Experimental Animal Center of the Jiangsu University. And db/db mice (female, 6–8 weeks old, 18–22 g) were purchased from the Cavens Experimental Animal Co., Ltd (Changzhou). They were provided free access to pellet food and kept water in plastic cages at 20 ± 2 °C and kept on a 12 h light/dark cycle in specific pathogen-free facilities. The experimental endpoints the animals were anesthetized euthanasia with pentobarbital sodium (Sprague-Dawley rat: 50 mg/kg, db/db mice: 30 mg/kg, Sigma-Aldrich) by intraperitoneal injection. Kidneys were fixed with 4% formaldehyde, embedded in paraffin, and sectioned to 4 μm thickness.
Cell culture
The rat renal tubular cells line NRK-52E was purchased from National Collection of Authenticated Cell Cultures. NRK-52E cells were maintained in DMEM (GIBCO, USA) containing 10% fetal bovine serum, 100 U mL−1 penicillin, and 100 mg mL−1 streptomycin in 5% CO2 at 37 °C.
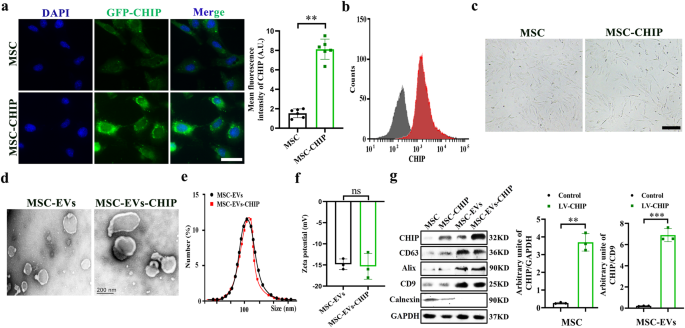
Mesenchymal stem cells were isolated from human umbilical cord according to a previously described method. In brief, take the umbilical cord from a full-term newborn, wash with PBS and cut off the arteries and veins. Cut it into 2 mm sized tissue blocks and stick them on a 6-well plate with an interval of 5 mm. Add basic DMEM (GIBCO, USA) medium containing 10% fetal bovine serum and 100 U mL−1 penicillin, and 100 mg mL−1 streptomycin in 5% CO2 at 37 °C. Thereafter, the medium was refreshed every 3 days and continuous culture until the purity of mesenchymal stem cells reached 85%.
Extraction and Purification of MSC-EVs-CHIP
A lentivirus vector encoding murine CHIP was purchased from genepharma (Suzhou, China). Mesenchymal stem cells were transfected with lentivirus encoding CHIP gene. When the transfection efficiency reached 80% measured by western blotting and flow cytometry analysis. The EVs in medium and FBS were depleted by ultracentrifugation. The medium was replaced and collected after 48 h. MSC-EVs were isolated and purified by ultracentrifugation33. In brief, the medium was centrifuged at 800 g for 10 min, 2000g for 10 min, 10 000 g for 30 min, 100,000 g for 70 min at 4 °C, to obtain MSC-EVs. The specific marker expression of CD63, CD9, CD81, and the CHIP expression was measured by western blotting.
Biological characterization analysis of MSC-EVs-CHIP
Prepared MSC-EVs and MSC-EVs-CHIP samples were pipetted onto a 300 meshes copper and incubated for 3 min. 20 µL of 1% uranyl acetate was pipetted onto the mesh copper and incubated for 1 min, washed and dry for TEM. The images were acquired using transmission electron microscope. MSC-EVs size determination by dynamic light scattering (DLS), in brief the volume of MSC-EVs and MSC-EVs-CHIP samples was increased to 1 mL with PBS and loaded into a quartz cuvette. The size distribution of EVs was measured by dynamic light scattering at 25 °C. Nanometer tracking analyzer detected its zeta potential and nanoparticle size.
Renal interstitial fibrosis model construction and treatment
RIF was established in Sprague-Dawley rats, anesthetize with inhalation of 2% isoflurane, expose the left ureter through a lateral incision, and ligated with double straps and surgical thread. The rats were randomly assigned into five experimental groups: Control, UUO, MSC-EVs, and MSC-EVs-CHIP, SPION-EVs. Rats were ureteral obstruction for 2 weeks, and intravenously injected with MSC-EVs, MSC-EVs-CHIP and SPION-EVs (2 mg per rat/every three days). After 2 weeks of treatment, the renal and blood serum were collected and analyzed renal function. The biodistribution of MSC-EVs-CHIP and SPION-EVs was performed a PerkinElmer IVIS Lumina II. In brief, MSC-EVs-CHIP and SPION-EVs was labeled with CM-DIR, and the free CM-DIR was washed thrice with cold PBS by ultracentrifugation for 3 times with cold PBS. Then, the CM-DIR-labeled EVs were injected intravenously into rat for 12 h and the CM-DIR signal in various tissues by PerkinElmer IVIS Lumina II.
RNA extraction and RT-qPCR
Trizol reagent was applied to extract total RNA from cultured cells or tissues in strict accordance with the instructions provided on the kit, followed by the determination of RNA concentration. mRNA was reverse-transcribed to cDNA and subjected to quantitative PCR, which was performed with the BioRad CFX96 Real-Time PCR Detection System (BioRad, USA). mRNA expression was compared using the 2−ΔΔCt relative quantification method, GAPDH was used as the endogenous control.
Western blot analysis
Renal tubular cells and kidney tissues were lysed using lysis buffer and the obtained protein lysates were quantitated by BCA assay, then degenerated at 100 °C for 5 min. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto polyvinylidene fluoride membranes and blocked with 5% bovine serum albumin. Membranes were immunoblotted with primary rabbit polyclonal antibodies to alpha-smooth muscle actin (α-SMA; 1:500, AB32575), Mothers against decapentaplegic homolog 2 (Smad2/3; 1:1000, CST3102), Fibronectin (FN, 1:1000, CST26836), Collagen I (Collagen I, 1:1000, CST72026) and incubated overnight at 4 °C with constant shaking. Next, the primary antibody-incubated membranes were washed five times with washing buffer and incubated with HRP-coupled secondary antibody for 1 h at room temperature. Protein bands were visualized by western blotting. Band intensity was quantified using the Image J software. The relative expression of the target protein was normalized to the band intensity of β-actin. We have included original western blot chemiluminescent images with corresponding light micrographs showing molecular weight markers for all western blots in Supplementary Fig. 10.
Histologic analysis
Renal tissues samples were fixed in 10% formalin solution, embedded in paraffin and sliced into 5μm thick sections. After deparaffinization and rehydration, sections were stained with hematoxylin and eosin (HE), Sirius red and Masson. The area at the junction of the cortex and medulla in the renal section was selected for histological analysis. HE staining was used to assess tubular injury, Sirius red and Masson staining was performed to evaluate the deposition of collagenous fibers in the renal interstitium. The positive area (red) of Sirius red and (blue) Masson staining was quantified using Image J.
Immunohistochemistry and Immunofluorescence
Paraffin-embedded tissues were heat-fixed, deparaffinized, rehydrated, antigen retrieved, and subsequent process. First, renal tissue sections were heat-fixed for 2 h at 60 °C and deparaffinized in xylene for 30 min, then rehydrated in 100%, 75%, and 50% ethanol respectively for 10 min. Antigen retrieval was performed using 10 × 10−3 M citrate antigen retrieval solution under high pressure for 30 min. The sections were incubated with 3% H2O2 for 10 min in the dark, blocked with 10% BSA for 1 h and stained with Smad2/3 and Fibronectin antibodies overnight at 4 °C, and washed three times with PBS. 50 µL HRP-labeled goat anti-mouse/rabbit Ig mixture was added to sections for 30 min and washed with PBST thrice. 50 µL DAB reagent was added for color reaction and restained with hematoxylin for 30 s. Finally, the sections were soaked in 50%, 75%, 100% ethanol, and xylene for 10 min respectively to dehydrate. The images of samples were acquired using Leica fluorescence optical microscope.
The renal sections after deparaffinized and rehydrated, then stained with Smad2/3, α-SMA, CD66, and CHIP, Slc5a12 antibodies overnight at 4 °C, and washed thrice times with PBS. The sections were stained with DAPI for confocal laser scanning microscopy (GE, USA). NRK-52E cells were washed twice with cold PBS. Then cells were fixed in 4% paraformaldehyde (PFA) for 10 min, blocked with 10% BSA for 1 h at room temperature, and stained with CHIP, Smad2/3, and α-SMA antibodies overnight at 4 °C. The cells were then washed thrice times with cold PBS, and stained with DAPI for confocal laser scanning microscopy (GE, USA).
Statistical analysis
All the experiments were randomized and blinded. All studies were performed in at least three independent experiments with each experiment including triplicate sets in vitro, or six animals per group in vivo. All data are presented as the mean ± SEM. GraphPad Prism 8.0 software (San Diego, CA, USA) was used for the statistical analysis. Western blotting and immunofluorescence analyses were performed using the Image J software. Data among multiple groups were compared using one-way analysis of variance, followed by a post hoc correction using Tukey’s test. a student’s t-test was used to test the difference between two groups. A value of *p < 0.05 was indicative of a statistically significant difference.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41536-024-00348-0
