Improvement of the protocol for generating expandable human liver organoids from iPSCs
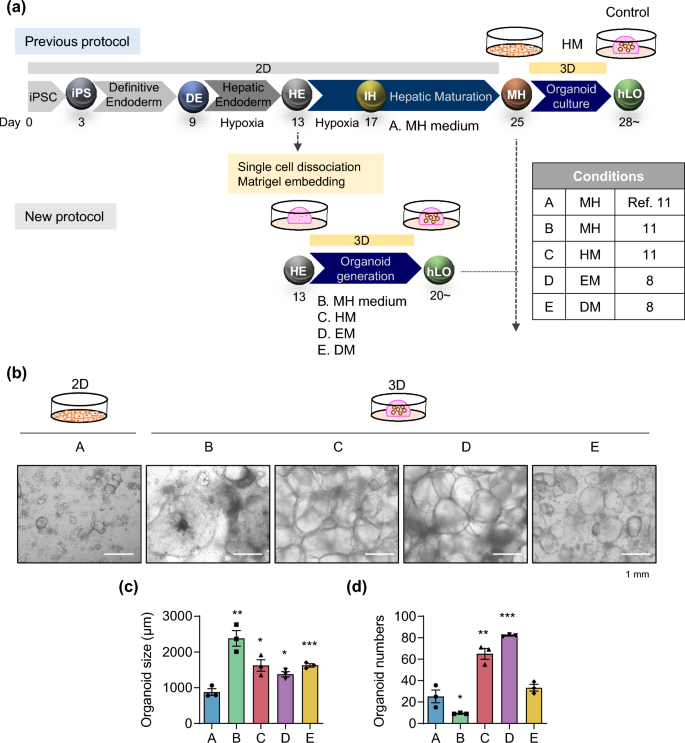
Previously, we developed expandable liver organoids from PSCs following the stage-specific liver developmental process from PSCs to definitive endoderm (DE), hepatic endoderm (HE), immature hepatocytes (IH), and mature hepatocytes (MH)11 (Fig. 1a; Previous protocol). However, the protocol requires more than 3 weeks of differentiation in a 2D format, generation efficiencies are highly variable between batches, and a complicated manual picking up process is needed. Therefore, to advance and standardize the protocol, we moved the organoid generation step forward to the HE stage. Cells were dissociated into single cells after completing HE differentiation and then embedded into Matrigel as a 3D format (Fig. 1a; New protocol). From the HE stage, the new protocol takes only one week to generate organoids, whereas the previous protocol takes more than two weeks. At this stage, we compared the following four previously reported types of medium for liver organoid generation including those of Takebe and Taniguchi’s group9, Clevers’ group8, and ours11: MH (mature hepatocyte medium in ref. 11, modified by Takebe’s protocol9) (Fig. 1a; A and B), HM (hepatic medium in ref. 11) (C), EM (expansion medium in ref. 8) (D), and DM (differentiation medium in ref. 8) (E) (Supplementary Table S1). At 25 days after starting differentiation, cell morphologies were compared (Fig. 1b). At this time point, organoids were enlarged in all 3D groups than in the 2D control group (Fig. 1b,c). The number of organoids was increased by 2.6-fold in HM (C) and by 3.3-fold in EM (D) compared with the 2D control (A) (Fig. 1d).
Efficient and reproducible generation of iPSC-derived liver organoids. (a) Schematic diagram of the previous (upper) and new (lower) protocols to generate iPSC-derived expandable liver organoids. A. MH (mature hepatocyte medium) (2D); B. MH (3D); C. HM (hepatic medium); D. EM (expansion medium); E. DM (differentiation medium). (b) Representative morphology of cells in each generation condition after 25 days of differentiation. (c) Organoid size and (d) organoid number in each condition after 25 days of differentiation. Data are the mean ± SEM (n = 3) and analyzed by the Student’s t-test. *p < 0.05, **p < 0.01, and ***p < 0.001.
Characterization of organoids generated using the new protocol
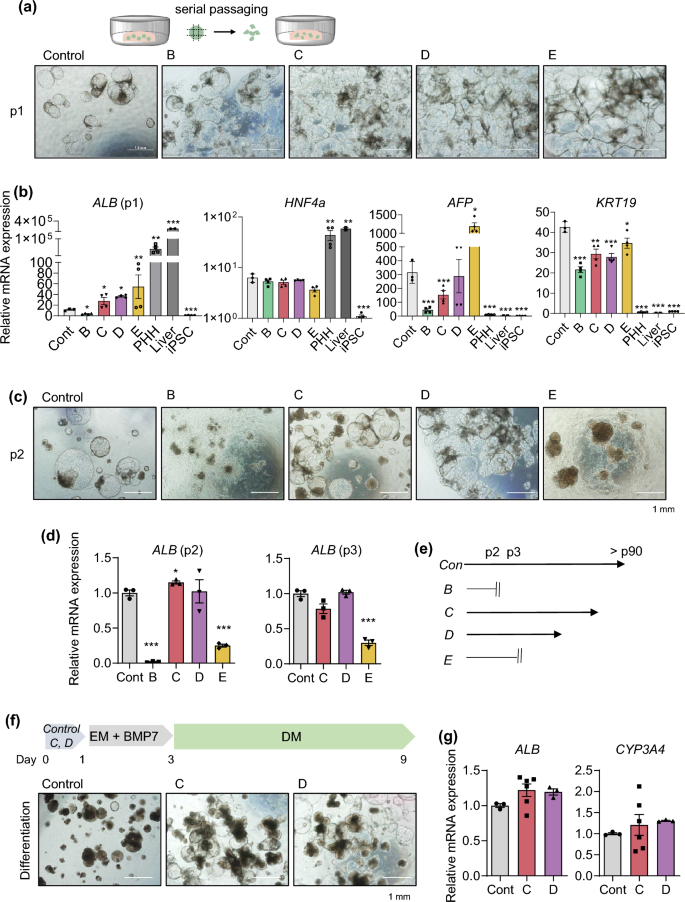
Next, to determine whether organoids generated under each condition could expand, we performed serial passaging by mechanically splitting the organoids using a blade and re-embedding them into fresh Matrigel (Fig. 2a). Albumin (ALB) gene expression was lowest in MH medium (B) and highest in DM medium (E) at passage 1 (Fig. 2b). However, ALB levels were significantly lower in both conditions (B and E) at passage 2 than in the control generated by the previous protocol (Fig. 2c,d). Importantly, further passaging was impossible beyond passage 2 in MH medium (B) and beyond passage 3 in DM medium (E) (Fig. 2c–e). Thus, further experiments could not be performed in either condition. On the other hand, organoids generated in HM (C) and EM (D) could be continuously passaged (Fig. 2c–e). Expression of hepatocyte markers such as ALB and hepatocyte nuclear factor 4 alpha (HNF4A) in both conditions was comparable with that in the control generated by the previous protocol (Fig. 2b,d). Their gene expression was substantially higher in the HM (C) and EM (D) conditions than in iPSCs, although it did not reach the levels observed in primary human hepatocytes (PHH) or human liver tissue (Fig. 2b). Expression of fetal liver/progenitor markers such as alpha-fetoprotein (AFP) and keratin 19 (KRT19) was rather lower in HM (C) and EM (D) than in the control generated by the previous protocol (Fig. 2b), indicating that earlier interaction with the 3D extracellular matrix can contribute to decrease the immaturity of iPSC-derived liver organoids. Moreover, expression of AFP was notably decreased under the HM (C) condition across passages and expression of the KRT19 gradually decreased until passage 4 and remained consistent thereafter (Supplementary Fig. S1). Conversely, expression of the mature hepatocyte marker cytochrome P450 3A4 (CYP3A4) and the epithelial cell marker EpCAM significantly increased as the passages numbers increased (Supplementary Fig. S1). Furthermore, when organoids generated using HM (C) and EM (D) were further differentiated under the sequential EM and DM condition8 (Fig. 2f), mRNA expression of ALB and CYP3A4 was compatible to that in the control (Fig. 2g). Like this, our previously defined novel medium, HM (C),11 is prominent in the potential of proliferation and differentiation without requiring expensive components such as R-spondin present in EM (D)8. Additionally, among 200 differentially expressed genes (DEGs, fold-change > 2, p value < 0.05) identified by RNA sequencing (RNA-seq) analysis comparing HM (C) and EM (D), 130 genes were up-regulated, and 70 genes were down-regulated (Supplementary Fig. S2a and Supplementary Table S2). Notably, genes associated with liver regeneration and proliferation, such as insulin like growth factor 2 (IGF2), insulin receptor substrate 2 (IRS2), insulin receptor (INSR), CCAAT enhancer binding protein delta (CEBPD)13, and intercellular adhesion molecule 1 (ICAM1)14,15, were up-regulated. Furthermore, gene sets related to cholesterol metabolism, the androgen response, and glycolysis were notably enriched in the HM (C) condition compared with the EM (D) condition, according to Gene Set Enrichment Analysis (GSEA) (Supplementary Fig. S2b). These pathways are predominantly enriched in liver tissue16,17,18,19,20, suggesting that the HM condition contributes to the maintenance of self-renewal and hepatic maturation potential in liver organoids. Thus, we next determined which components of HM are essential for long-term expansion of liver organoids.
Characterization of organoids generated by the new protocol. (a) Representative morphology of organoids in each condition at passage 1 (p1). (b) mRNA expression levels of the indicated genes in each condition at p1. Primary human hepatocytes (PHH) and human adult liver tissue samples were used as positive controls and human iPSCs were used as a negative control. (c) Representative morphology of organoids in each condition at p2. (d) mRNA expression levels of ALB in each condition at p2 and p3. (e) Passages to which it was possible to expand in each condition. (f) Scheme of differentiation (upper) and representative morphology of the organoids after differentiation (lower). (g) mRNA expression levels of ALB and CYP3A4 in each condition of the organoids after differentiation. Data are the mean ± SEM (n = 3) and analyzed by the Student’s t-test. *p < 0.05, **p < 0.01, and ***p < 0.001.
Characterization of the components of HM required for long-term culture
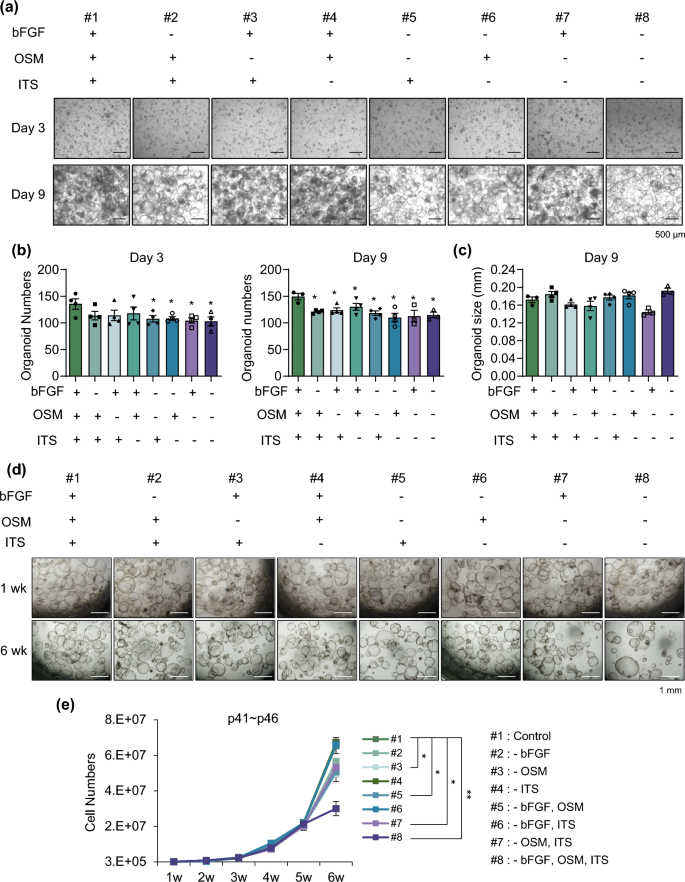
As shown in Supplementary Table S1, basic fibroblast growth factor (bFGF), oncostatin M (OSM), and insulin-transferrin-selenium (ITS) are specific components of HM different from other medium. Therefore, we excluded each component individually and in combination from HM during organoid generation (Fig. 3a). The organoid number was slightly, but not significantly, decreased when only one factor was removed at 3 days after seeding (#2-#4), indicating that the other factors compensate during the early phase of organoid generation (Fig. 3b, left). However, when two (#5-#7) or all three (#8) factors were removed, the organoid number was decreased at this time point (Fig. 3b, left). At 9 days after seeding, the final organoid number was decreased in all groups (Fig. 3b, right). However, organoid size was not affected by elimination of bFGF, OSM, or ITS at 9 days after seeding, since after organoids were generated (Fig. 3c). Next, we determined the effect of removal of these factors on expansion of late passage organoids during long-term passaging (Fig. 3d). Organoids at passage 40 were expanded for 6 weeks without bFGF, OSM, or ITS. The cell number was significantly decreased in conditions #3 (-OSM), #5 (-bFGF, OSM), and #7 (-OSM, ITS) at 6 weeks of culture, and organoid proliferation was potent inhibited under condition #8, in which all three factors were removed (Fig. 3e). Interestingly, upon OSM removal, the population of smaller organoids significantly increased (Supplementary Fig. S3). Specifically, the percentage of small organoids (< 200 μm) was significantly increased in conditions #3, #5, #7, and #8. These data suggest that bFGF, OSM, and ITS in HM are essential for efficient generation and long-term expansion of organoids. Specifically, OSM plays a crucial role in the growth of organoids during late passages.
Essential factors in the medium for long-term culture. (a) Representative morphology of organoids in each condition on day 3 (upper) and day 9 (lower) after depletion. (b) Organoid number in each depleted condition on day 3 (left) and day 9 (right). (c) Organoid size in each depleted condition on day 9. (d) Representative morphology of late passage organoids in each condition at 1 week (upper) and 6 weeks (lower) after depletion. (e) Cell number of late passage organoids after serial passaging in each indicated depleted condition. Data are the mean ± SEM (n = 3) and analyzed by the Student’s t-test. *p < 0.05 and **p < 0.01.
Molecular characteristics of proliferative liver organoids
To define the molecular characteristics of proliferative liver organoids in HM, we compared their transcriptome profiles with those of non-proliferative cells in MH condition by RNA-seq (Supplementary Fig. S4). Top hallmark enriched pathways in HM compared with MH were mainly involved in two groups of gene sets: functional maturation (oxidative phosphorylation, fatty acid metabolism, and bile acid metabolism) and proliferation (E2F targets, MYC targets, and G2M check point) of organoids (Supplementary Fig. S4a). Representative top-ranked subsets corresponding to the two groups in GSEA are shown in Supplementary Fig, S4b. Next, DEGs whose expression was changed more than twice as much in HM compared with that in MH were selected (Supplementary Fig. S4c). The top ten ranked biological processes determined by gene ontology (GO) analysis included metabolic processes of lipids, organic acids, and monocarboxylic acids; homeostatic process; secretion; ion transport; and cell proliferation (Supplementary Fig. S4d). mRNA expression of representative genes corresponding to functional maturation was confirmed by real-time PCR (Supplementary Fig. S4e). Expression of genes related to lipid and bile acid metabolism was significantly higher in HM condition compared to MH (Supplementary Fig. S4e).
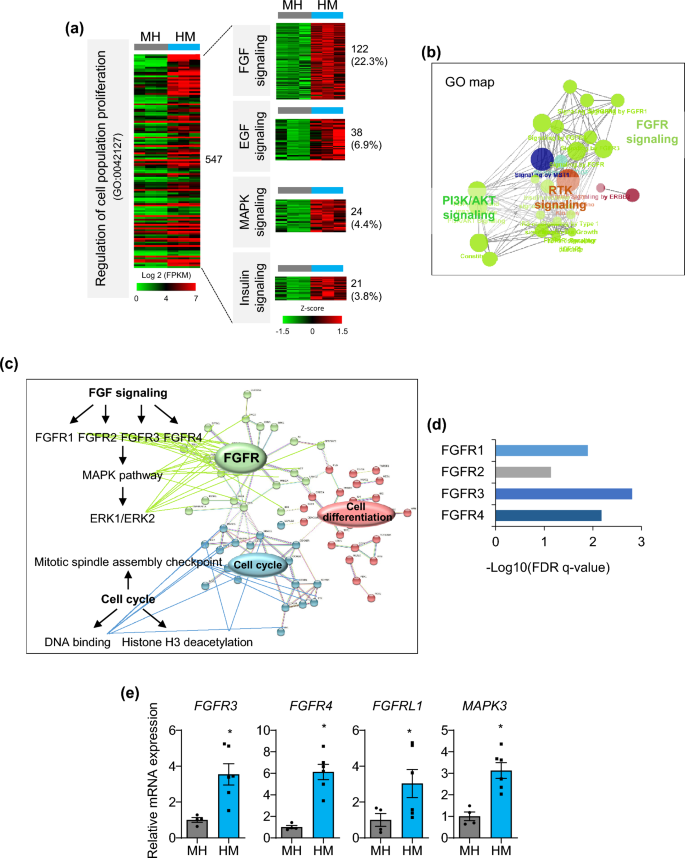
Notably, proliferation-related genes (GO:0,042,127) accounted for approximately one-quarter of DEGs (547 of 2,142 DEGs (Supplementary Fig. S4c)) between MH and HM (Fig. 4a). Around 37% of them were associated with growth signaling such as FGF (22.3%), epidermal growth factor (EGF) (6.9%), mitogen-activated protein kinase (MAPK) (4.4%), and insulin (3.8%) signaling (Fig. 4a. Consistently, gene sets related to FGFR, Phosphoinositide 3-kinases (PI3K)/AKT, and receptor tyrosine kinases (RTK) signaling were enriched in the GO map (Fig. 4b). FGF signaling is the main pathway for hepatocyte proliferation in liver development and regeneration21, therefore, we analyzed the protein–protein interaction (PPI) network construction (Fig. 4c) and performed Reactome pathway analysis (Fig. 4d) focusing on FGF signaling (122 genes). High confidence interacting partners were FGFR signaling (FGFR, MAPK, and ERK pathways), regulation of the cell cycle (mitotic spindle assembly checkpoint, DNA binding, and histone H3 deacetylation), and cell differentiation (epithelial cell differentiation, bile duct development, and tube development) (Fig. 4c). In particular, FGFR3 and FGFR4 were highly enriched in HM condition (Fig. 4d). Real-time PCR confirmed that mRNA expression of FGFR3, FGFR4, FGFRL1, and the FGF signaling down-stream gene, MAPK3, was significantly increased in HM (Fig. 4e), indicating that FGFR signaling may be the major pathway that maintains the proliferative capacity of liver organoids in HM condition.
Top enriched pathways of proliferative liver organoids. (a) Heat map of the ‘regulation of cell population proliferation’ GO term (left) in DEGs between MH and HM and growth-related signaling within them (right). (b) GO map of ‘regulation of cell population proliferation’ genes enriched in HM. (c) PPI network analysis of 122 genes involved in FGF signaling. (d) Top enriched pathways in FGF signaling by Reactome analysis. (e) mRNA expression levels of genes related to major growth signaling in MH and HM condition. Data are the mean ± SEM (n = 3) and analyzed by the Student’s t-test. *p < 0.05.
Impaired generation and proliferation of liver organoids upon knockdown of FGFR4
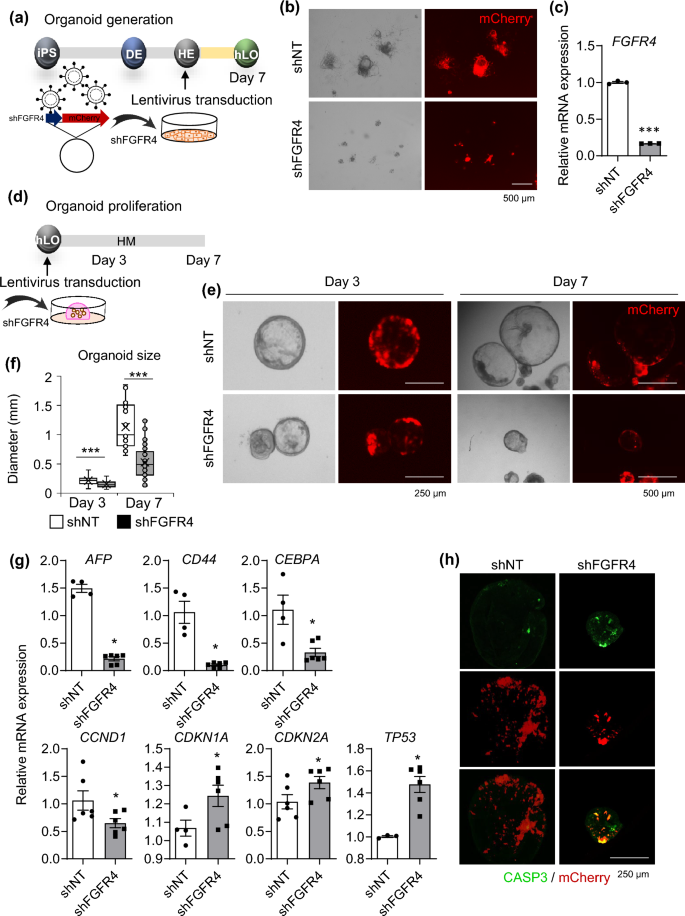
Next, we confirmed the effect of FGFR4 knockdown by lentiviral short hairpin RNA (shRNA) transduction on the generation and proliferation of liver organoids (Fig. 5), because siRNA-mediated Fgfr4 knockdown impairs hepatocyte proliferation in mice21. First, cells were dissociated into single cells at the HE stages during organoid generation and then transduced with shRNA targeting FGFR4 (shFGFR4) or non-targeting control shRNA (shNT) (Fig. 5a). At 7 days after transduction, strong mCherry expression was detected in both groups, but shFGFR4-transduced cells failed to generate organoids (Fig. 5b and Supplementary Fig. S5a). FGFR4 expression was downregulated in shFGFR4-transduced cells compared with shNT-transduced control (Fig. 5c). shFGFR4 transduction during organoid generation also significantly down-regulated mRNA expression of FGFR4-related genes such as FGFR3, FGFRL1, and MAPK3 (Supplementary Fig. S5b); the cell cycle progression regulator, CCND1; and important factors for hepatic regeneration such as AFP, LGR5, CD44, CEBPA, and PROM1 (Supplementary Fig. S5c). Second, HM-cultured proliferative organoids were transduced with shFGFR4 (Fig. 5d). Organoid growth was slow at 3 days after transduction and organoid size was prominently decreased at 7 days after transduction (Fig. 5e,f). shFGFR4 transduction potently downregulated mRNA expression of hepatic regeneration-related genes (AFP, CD44, and CEBPA) and CCND1, but upregulated expression of cell cycle inhibitor genes such as CDKN1A, CDKN2A, and TP53 (Fig. 5g). Furthermore, caspase 3-positive apoptotic cells were prominently detected in organoids at 3 days after transduction of shFGFR4 (Fig. 5h). These results demonstrate that FGFR4 signaling is critical for the generation and maintenance of proliferative liver organoids.
Effects of FGFR4 knockdown on generation and maintenance of organoids. (a) Schematic diagram of lentiviral transduction of shFGFR4 during organoid generation. (b) Representative morphology (left) and mCherry expression (right) of cells transduced with shNT (upper) and shFGFR4 (lower) at 7 days after transduction. (c) mRNA expression levels of FGFR4 in shNT- and shFGFR4-transduced cells. (d) Schematic diagram of lentiviral transduction of shFGFR4 in HM-cultured proliferative organoids. (e) Representative morphology and mCherry expression of shNT- and shFGFR4-transduced organoids at 3 days (left) and 7 days (right) after transduction. (f) Organoid size in each condition at 3 and 7 days after transduction. (g) mRNA expression levels of the indicated genes in shNT- and shFGFR4-transduced cells at 3 days after knockdown. (h) Immunostaining of CASP3 (green, upper), mCherry signals (red, middle), and merged images (bottom) in each condition at 3 days after knockdown. Data are the mean ± SEM (n = 3) and analyzed by the Student’s t-test. *p < 0.05 and ***p < 0.001.
Furthermore, we previously developed a Liver-specific Gene Expression Panel (LiGEP) that includes 93 genes expressed only in human adult liver tissue22 and used it as a validation platform to quantitatively evaluate the similarity between liver tissue and organoids generated from iPSCs11. Genes expressed in HM, but not in MH, were 20 from 93 genes (Supplementary Fig. S6a). These genes included those encoding the liver-specific glucose transporter, solute carrier family 2 member 2 (SLC2A2), drug-metabolizing enzymes (CYPs and UGTs), bile transport and synthesis enzymes (SLCO1B1 and BAAT), and plasma proteins secreted by the liver (SERPINA4, HABP2, and F12). Importantly, genes encoding several regeneration-related factors such as CYP8B1, SLC38A4, CXCL2, and TAT were expressed specifically in HM. The mRNA expression levels of these enriched genes in HM were confirmed by real-time PCR (Supplementary Fig. S6b). Overall, these data demonstrate that liver organoids generated and cultured in HM condition may acquire the functional maturation capacity and regenerative and proliferative potential of the liver. Therefore, we next applied our novel organoid generation protocol to model the genetic liver disease GSD1a.
Generation of liver organoids from GSD1a patient-derived iPSCs and their characterization
To acquire GSD1a patient-specific iPSCs, commercially available patient fibroblasts23 were reprogrammed using Sendai viruses (Supplementary Fig. S7a and b). Pluripotency of the established GSD1a patient-derived iPSCs was determined by alkaline phosphatase (AP) staining (Supplementary Fig. S7c) and by mRNA expression analysis (Supplementary Fig. S7d) and immunostaining (Supplementary Fig. S7e) of pluripotency markers. The differentiation potential was confirmed by immunostaining of markers of the three germ layers after in vitro differentiation through embryoid body (EB) formation (Supplementary Fig. S7f.). GSD1a patient-derived iPSCs maintained a normal karyotype after iPSC generation (Supplementary Fig. S7g).
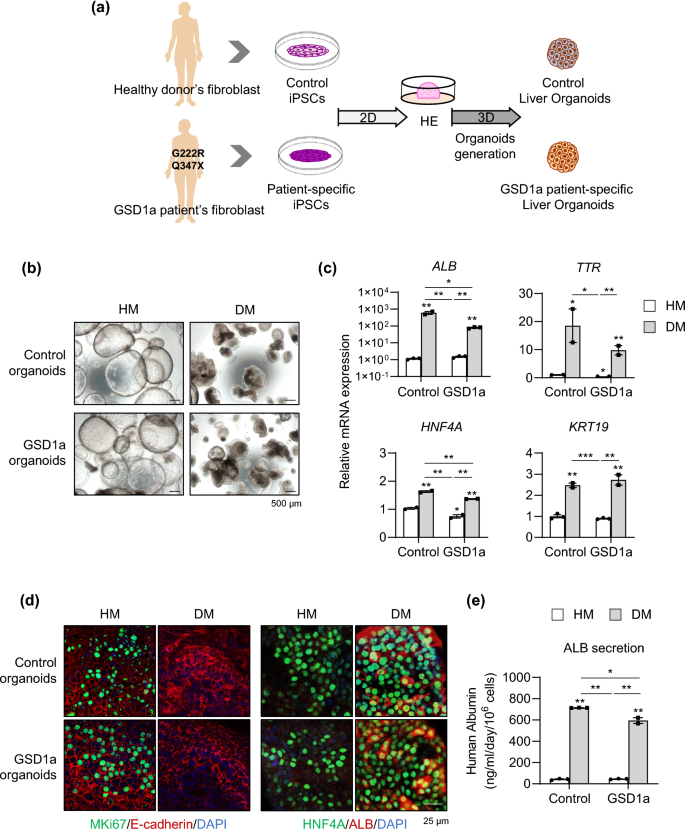
Next, liver organoids were generated from iPSCs of a healthy control and a GSD1a patient using the new protocol (Fig. 6a). GSD1a organoids were well generated and did not morphologically differ from control cells at each stage of differentiation during organoids generation (Supplementary Fig. S8a). Serial passaging was possible so far beyond passage 31 (Supplementary Fig. S8b), indicating the scalability and reproducibility of our new protocol. After 6 days of further differentiation in DM condition (Fig. 6b), GSD1a organoids had slightly lower levels of ALB, TTR, and HNF4A expression, but there were no remarkable differences compared with control organoids (Fig. 6c). MKI67-positive proliferating cells disappeared upon differentiation, and HNF4A and ALB protein expression (Fig. 6d) and ALB secretion (Fig. 6e) were prominently detected in both differentiated control and GSD1a organoids. These results demonstrate that liver organoids can be efficiently and reproducibly generated from GSD1a liver disease patient cells as well as healthy donor cells using the new protocol.
Generation of liver organoids from GSD1a patient-derived iPSCs by the new protocol and their characterization. (a) Schematic diagram of liver organoid generation from healthy donor control and GSD1a patient-derived iPSCs using the new protocol. (b) Representative morphology of control (upper) and GSD1a (lower) organoids in HM and DM condition. (c) mRNA expression levels of the indicated genes in control and GSD1a organoids in HM and DM conditions. (d) Immunostaining of control and GSD1a organoids in HM and DM condition with the indicated antibodies. (e) Quantification of ALB secretion by control and GSD1a organoids in HM and DM condition. Data are the mean ± SEM (n = 3) and analyzed by the Student’s t-test. *p < 0.05, **p < 0.01, and ***p < 0.001.
Disease modeling of GSD1a using patient-specific liver organoids generated by the new protocol
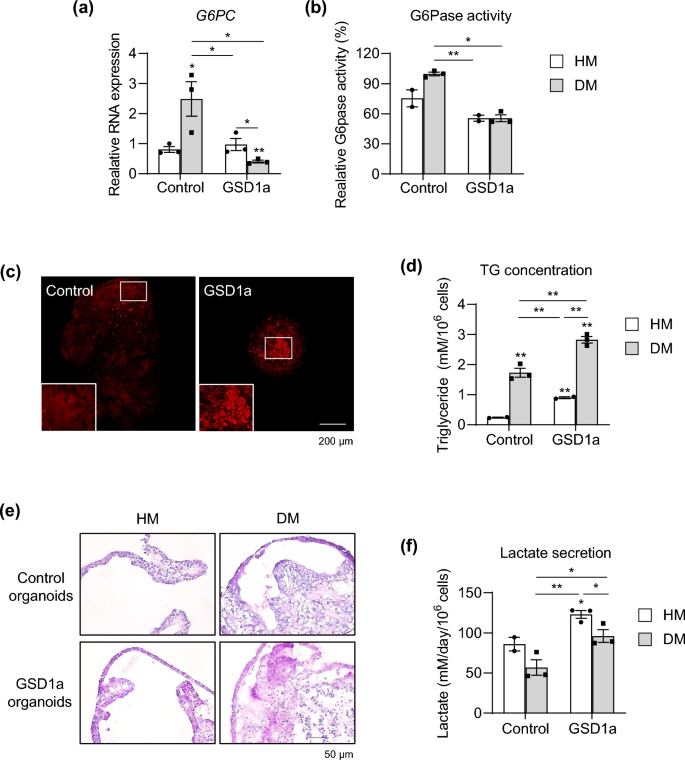
To characterize disease phenotypes of the generated patient iPSC-derived liver organoids, G6PC mRNA expression (Fig. 7a) and G6Pase enzyme activity (Fig. 7b) were determined. mRNA expression and enzyme activity were substantially decreased by 83.5% and 43%, respectively, in GSD1a organoids compared with control organoids in DM condition (Fig. 7a,b). Moreover, accumulated natural lipids detected by Nile red staining were distinctly observed in GSD1a organoids (Fig. 7c). Quantitatively, the triglyceride (TG) concentration was increased over 1.9-fold in GSD1a organoids compared with that in control organoids in DM condition (Fig. 7d). In addition, periodic acid-Schiff (PAS) staining revealed that glycogen strongly accumulated in GSD1a organoids (Fig. 7e). Moreover, increased lactate secretion of GSD1a organoids was easily detected even in medium both from HM- and DM-cultured organoids (Fig. 7f). Taken together, these results demonstrate that liver organoids generated by the new protocol well maintain patient-specific disease phenotypes, and their scalability and practicality may facilitate their application as an organoid platform for personalized disease modeling and drug screening for treatments of liver diseases.
Disease modeling using GSD1a patient-specific liver organoids. (a) mRNA expression levels of G6PC in control and GSD1a organoids in HM and DM conditions. (b) G6Pase enzyme activity in control and GSD1a organoids in HM and DM condition. (c) Lipid droplets stained with Nile red in control and GSD1a organoids in DM condition. (d) Quantification of TG in control and GSD1a organoids in HM and DM condition. (e) Glycogen accumulation detected by PAS staining in control (upper) and GSD1a (lower) organoids in HM and DM conditions. (f) Quantification of lactate secretion by control and GSD1a organoids in HM and DM condition. Data are the mean ± SEM (n = 3) and analyzed by the Student’s t-test. *p < 0.05, **p < 0.01, and ***p < 0.001.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-023-50250-w