The present study was conducted at the delivery room and in the Obstetrics & Gynecology department in Mansoura University Hospital (MUH). All necessary Maternal data were obtained from medical records (Such as name, age, occupation, residency, gravidity, parity, medical history, pregnancy related complications, blood pressure, blood glucose monitoring during pregnancy, antenatal screening for various diseases and serologic results for blood transmitted diseases (syphilis, Hepatitis B virus (HBV), Hepatitis C virus (HCV), Human Immunodeficiency Virus (HIV), maternal blood group, ABO and Rh incompatibility, maternal hemoglobin and need for blood transfusion antenatal or intra-partum and use of antenatal steroids.
The indication of elective cesarean section (ECS) and Doppler evaluation of the placenta and the cord were revised. Potentially eligible mothers were approached upon arrival and written informed consent was obtained before enrollment in the study after a thorough explanation of the procedure.
Study designs
This was a cross sectional study with analytic components.
Participants
Random selection of 103 eligible newborn babies were enrolled in the study.
Inclusion criteria
Full term babies (gestational age 37–40 weeks), delivered by elective cesarean section (ECS) and didn’t require more than supportive care (vigorous babies with normal heart rate and breathing) were eligible for the study.
Exclusion criteria
Preterm newborns (gestational age less than 37 weeks), multiple gestation and babies who required major resuscitative measures along with presence of any maternal illnesses as diabetes or hypertension or blood transmitted diseases (syphilis, HBV, HCV, HIV) were excluded. Additionally, those with placental abnormalities and intrauterine growth restriction (IUGR) as evident by ultrasound fetal biometry and uterine Doppler studies, where delay of resuscitation measures was not possible, were also excluded from the study.
Intervention
The intervention was to delay clamping of the umbilical cord (30 s or 60 s after delivery). All aspects of obstetric and neonatal care were managed according to the standard protocol of MUH, Mansoura, Egypt. All staff in the delivery unit were fully aware of the study procedure before the trial started.
Immediately after transferring the baby to the adjacent ordinary care unit, every baby went through all steps of newborn ordinary care, dried thoroughly under radiant warmer (servo control), maintained in sniffing position with brief gentle suction as needed. The time from complete delivery of the baby to the first clamp applied on the umbilical cord was measured with a stopwatch by the assistant. Consequently, the umbilical cord was sterilized with 70% alcohol swabs. Then, two ml of cord blood were withdrawn within 2–5 min after delivery by using wide bore needle in K2 EDTA tube (Greiner Bio One), then gently mixed by inverting the tube 5–10 times and placed on a rocker for up to 30 min, and eventually refrigerated at 2–8 °C. Clotted samples were discarded.
The collected samples were analyzed at Mansoura Research Center for Cord Stem Cells (MARC-CSC). Samples had to be analyzed within 12 h of blood collection to ensure viability of stem cells. Neonatal data such as gestational age, gender and birth weight were recorded in data sheets.
Outcomes
The primary outcome was to measure the total nucleated cells (TNC) and peripheral blood percentage of cluster of differentiation 34 (CD34+) in cord blood sample as a marker of stem cell level. The TNC count which was determined using the automated cell counter, sysmex XS-800i cell counter (Sysmex Corporation, JAPAN) and the number of hematopoietic stem cells CD34+ which was evaluated by flow cytometric analysis by BD Accuri™ C6 Cytometer (Becton, Dickinson, and Company).
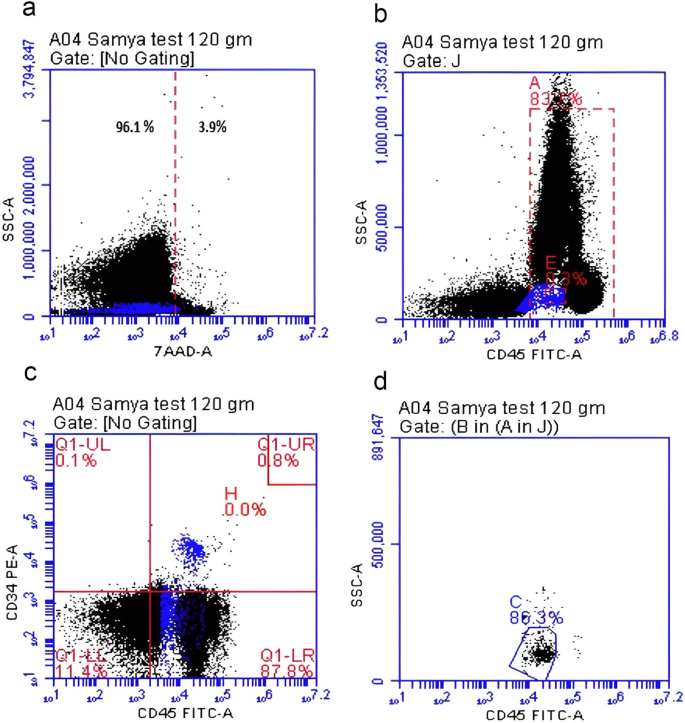
The data were analyzed using the software System (Becton, Dickinson, and Company). The Stem-Kit™ Reagents (Beckman Coulter, USA) were used. Histograms were used to characterize CD34 + /CD45 + dim hematopoietic stem cells by Flow Cytometry according to the International Society of Hematotherapy and Graft Engineering (ISHAGE) Guidelines18. The clinical pathologist and the technical person who measured the HPCs were blinded regarding the study groups. Gating was done in four steps as illustrated in Fig. 1. (a) Identification of cell viability using 7-Amino-Actinomycin D (7-AAD), a fluorescent intercalator dye that can selectively bind to DNA in dead cells. (b) Gating of live CD45 + hematopoietic cells; (c) Gating of double positive cells CD45 + /CD34 + cells; (d) Identification of the hematopoietic stem cells population CD34 + /CD45 + dim cells. The absolute count of CD34+ hematopoietic stem cells was calculated by multiplying the obtained percentage of CD34+ by the absolute TNC/μL as per ISHAGE Guidelines18.
Gating and detection of CD34+ cells by flowcytometry. (a) Identification of cell viability using 7-Amino-Actinomycin D (7-AAD), a fluorescent intercalator dye that can selectively bind to DNA in dead cells, (b) Gating of live CD45 + hematopoietic cells (83% of viable cells), (c) Gating of double positive cells CD45 + /CD34 + cells (0.8% of cells), (d) Identification of the hematopoietic stem cells population CD34 + /CD45 + dim cells (86.3% of double positive for CD45+ and CD34+).
Power of the study
Power was calculated by the Stata Statistical Software (Stata Corp LLC, 2021, Release 17. College Station, TX). In the current study, the newborn total blood count in group 1 was 14672 ± 6098.2 cells/µL, while the total blood count in group 2 was 20366 ± 19479.8 cells/µL. Thus, using the Wilcoxon- Mann Whitney model, with power of 83.5% and α error 5%, we aimed to enroll 50 participants per group to detect the statistical difference between two groups.
Randomization
When delivery was imminent (expected within 10 min), the nurse opened a sealed, numbered, opaque envelope containing the treatment allocation deigned by random allocation sequence generated by Microsoft Excel19. The included babies were randomly assigned into 2 groups: Group 1: babies were subjected to DCC 30 s after birth (50 newborns), Group 2: babies were subjected to DCC 60 s after birth (53 newborns).
Statistical analysis
Statistical analysis was performed using commercially available software, Statistical package for Social Science (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). Mann Whitney Test was used to assess the statistical significance of the non-parametric variable difference between two study groups. Chi-Square test was used to examine the relationship between two qualitative variables. Fisher’s exact test was used to examine the relationship between two qualitative variables when the expected count is less than 5 in more than 20% of cells.
Correlation was done using Spearman’s correlation. Linear regression analysis was used for prediction of risk factors, using generalized linear models. Univariate regression was used to examine the effect of a single independent variable while in multivariate regression analysis, more than one variable is analysed together for association or interactions, to explore which of the independent variables are independently associated with the outcome. All reported p values were two-tailed and p < 0.05 was considered statistically significant.
Ethics approval and consent to participate
This study was in line with the principles of the Declaration of Helsinki and approved by Mansoura Faculty of Medicine Institutional Research Board (MS/17.02.80). We obtained informed consents from all mothers prior to delivery.
Consent to participate
Written informed consent was obtained from mothers prior to inclusion of their babies and cord blood sampling in the study.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-023-50100-9