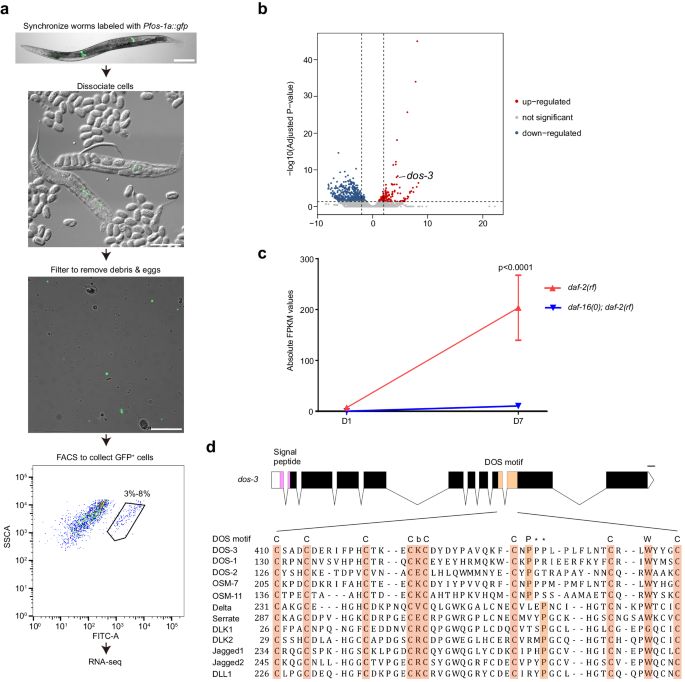
RNA-seq transcriptional profiling to define the DAF-16/FOXO transcriptomes of isolated adult PSG cells
We speculated that a secreted signal, whose expression is controlled by DAF-16/FOXO, mediates the effect of PSG DAF-16/FOXO activity on the distal GSPC pool. To identify relevant DAF-16/FOXO targets, we performed a PSG-specific gene profiling analysis by extracting RNA from isolated PSG cells of a daf-2-reduction-of-function (rf) mutant, daf-2(e1370) (high DAF-16/FOXO activity) and a daf-16(0); daf-2(rf) double mutant with a null allele of daf-16, mu86 (no DAF-16/FOXO activity). To mark cells of the PSG, we obtained a MosSCI insertion that expresses GFP under a PSG-specific promoter14 (Pfos-1a::gfp) and crossed this transgene into the daf-2(rf) and daf-16(0); daf-2(rf) backgrounds. For cell dissociation, worms were treated with proteinases and mechanically disrupted. After dissociation, GFP+ cells were immediately isolated by fluorescence-activated cell sorting (FACS). RNA was extracted from the collected cells and used for RNA-seq experiments (Fig. 1a, Supplementary Fig. 1). The DAF-16/FOXO transcriptomes of isolated young and aging PSG cells were determined by identifying genes that were differentially expressed in the PSG cells of daf-2(rf) and daf-16(0); daf-2(rf) animals on day 1 (D1) and day 7 (D7) of adulthood, respectively. The complete dataset is available in the Gene Expression Omnibus (GEO) database under accession code GSE243994.
a Flow chart for RNA-seq transcriptional profiling of isolated adult C. elegans PSG cells. Scale bars, 100 μm. b Volcano plot comparing mRNAs identified by RNA sequencing of D7 daf-2(rf) and daf-16(0); daf-2(rf) PSG cells. Red and blue dots represent genes up-regulated and down-regulated in daf-2(rf) PSG cells, respectively. c RNA-seq data of dos-3. Its expression was up-regulated in D7 daf-2(rf) PSG. Data are represented as mean ± SEM. n = 3 biological replicates. P value is calculated using Benjamini-Hochberg test. Source data are provided as a Source Data file. d Top: schematic showing the genomic structure of dos-3. Graph generated using Exon-Intron Graphic Maker (http://www.wormweb.org/exonintron). Solid boxes, exons; empty boxes, UTRs; lines, introns. The conserved signal peptide and DOS motif are highlighted in magenta and beige, respectively. Scale bar, 100 bp. Bottom: DOS motif-containing sequences from C. elegans (DOS-3, DOS-1, DOS-2, OSM-7, and OSM-11), Drosophila (Delta and Serrate), and human (DLK1, DLK2, Jagged1, Jagged2, and DLL1) proteins are aligned under the DOS motif consensus: C-X(3)-C-X(3,8)-C-X(2,5)-C-b-C-X(10,12)-C-X(1,3)-P-X(6,9)-C-X(1,4)-W-X(1,4)-C. Conserved DOS-motif amino acids are shaded. b represents K, V, E, or R, and the asterisks indicate possible positions for proline in the DOS motif.
We compared our D1 PSG-specific DAF-16/FOXO transcriptome with the previously reported D1 whole-worm DAF-16/FOXO transcriptome15,16. Our dataset was much smaller and although overlap between the two gene lists was observed, most of the PSG DAF-16/FOXO-regulated genes differed from those identified in the whole-worm set (Supplementary Fig. 2a). This result is consistent with our estimation from the FACS data that PSG cells comprise only 3-8% of the cell population in the adult C. elegans hermaphrodites, therefore expression changes of relevant DAF-16/FOXO targets in the PSG could be masked by their expression in other tissues. During the reproductive period of C. elegans hermaphrodites, cells in the PSG play an important role in regulating oocyte maturation, ovulation, and fertilization17,18. These processes require normal IIS19. Consistently, pathway enrichment analysis of our D1 PSG-specific DAF-16/FOXO transcriptome showed that gene clusters associated with these PSG functions such as “calcium ion transmembrane import into cytosol”, “striated muscle contraction”, and “oogenesis” were overrepresented, confirming that our cell isolation method is highly selective for PSG cells (Supplementary Fig. 2b).
In line with the timing of unmated hermaphrodites’ reproductive cessation on ~D5, our D7 PSG-specific DAF-16/FOXO transcriptome was no longer enriched with gene classes associated with PSG functions in reproduction, but with categories involved in stress responses including “mitochondrial unfolded-protein response”, “FoxO signaling pathway”, as well as “response to heat” (Supplementary Fig. 2c). This up-regulation of stress response genes in the aging daf-2(rf) PSG suggests that an anti-aging response is elicited in these cells, which could lead to the production of an intercellular signaling molecule to modulate progenitor activity in the distal germ line.
Analysis of the aging PSG-specific DAF-16/FOXO transcriptome identifies dos-3
While examining our aging PSG-specific DAF-16/FOXO transcriptome, we found a gene dos-3, representing a particularly attractive candidate for the signal from PSG to the distal germ line in the daf-2(rf) animals for the following reasons: (1) its expression was up-regulated by DAF-16/FOXO activity in the aging daf-2(rf) PSG (Fig. 1b, c); (2) it bears a signal peptide sequence at its N-terminus suggestive of a secreted protein (Fig. 1d); (3) it encodes a putative non-canonical Notch ligand. DOS-3 and related C. elegans proteins (DOS-1, DOS-2, OSM-7, and OSM-11) do not contain the characteristic DSL domain of canonical Notch ligands, but instead possess a conserved DOS motif that is present in the extracellular domain of many known Notch ligands in other species (Fig. 1d, Supplementary Fig. 3). Although the function of DOS-3 was not characterized, two other DOS proteins, OSM-7 and OSM-11, had been shown to be able to activate LIN-12 and GLP-1, the two Notch receptors in C. elegans, to regulate biological processes such as vulval development, adult chemosensory response, and larval molting quiescence20,21. We therefore hypothesized that DOS-3 might be the signal from PSG that could act upon the GLP-1/Notch signaling in the distal germ line to affect GSPC maintenance over time.
dos-3 is up-regulated in aging daf-2(rf) PSG
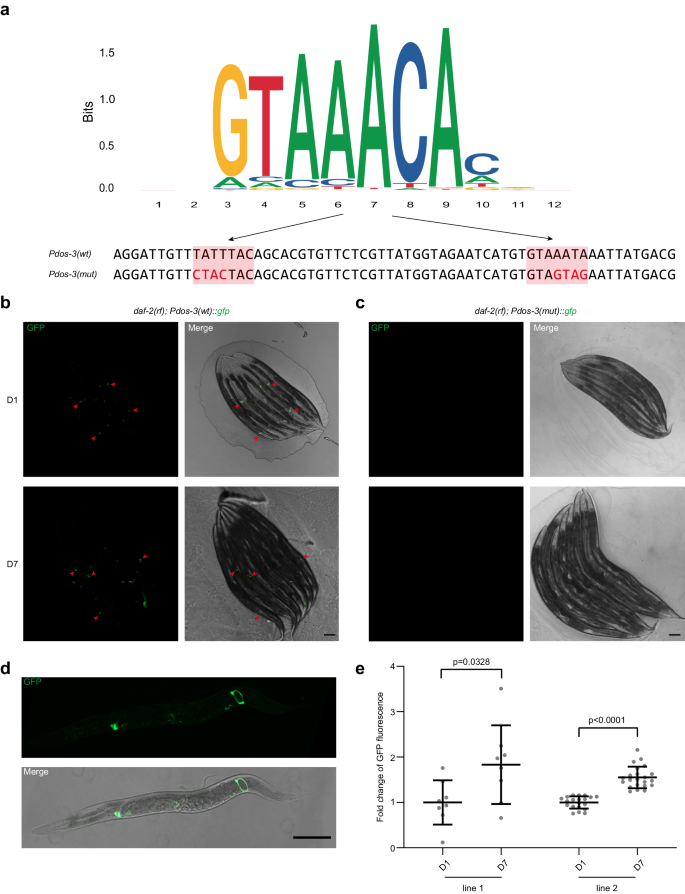
To define the expression pattern of dos-3 during aging, we generated two transcriptional reporters of dos-3 by expressing gfp under the control of its endogenous promoter using extrachromosomal arrays (Fig. 2a). We observed similar results for both transgenic lines: GFP fluorescence was detected in the PSG cells of daf-2(rf) animals on both D1 and D7 (Fig. 2b, d); additionally, we observed a significant increase in GFP fluorescence intensity in D7 daf-2(rf) PSG cells relative to D1, confirming that dos-3 is up-regulated in aging daf-2(rf) PSG (Fig. 2e).
a Top: consensus DBE sequences identified from C. elegans ChIP-seq data by Jaspar. Bottom: DBE-containing sequences of the dos-3 promoters used in our transcriptional reporter analysis. The two DBEs are shaded in coral with the mutated bases indicated in red. b, c Expression of dos-3 transcriptional reporters in daf-2(rf). Worms are oriented head toward the upper left corner. Scale bars: 100 μm. b Representative GFP and merge images of D1 and D7 daf-2(rf); Ex[Pdos-3(wt)::gfp] worms. Arrowheads indicate GFP expression in the PSG cells. c Representative GFP and merge images of D1 and D7 daf-2(rf); Ex[Pdos-3(mut)::gfp] worms. GFP is undetectable in these animals. d PSG expression of Pdos-3(wt)::gfp in daf-2(rf). Representative GFP and merge images of an adult daf-2(rf); Ex[Pdos-3(wt)::gfp] worm. The worm is oriented head to the left. Scale bar: 100 μm. e Fold change of GFP fluorescence in D7 daf-2(rf); Ex[Pdos-3(wt)::gfp] worms relative to D1. e is a quantification of data represented in (b). Two transgenic lines were examined for this experiment. Data are represented as mean ± SD with individual values shown as dots. n = 8, 8, 20, 22 animals from left to right. P values are calculated using two-tailed Student’s t-test. Source data are provided as a Source Data file.
DAF-16/FOXO binds to dos-3 promoter
DAF-16/FOXO induces expression of its canonical targets by binding to the DAF-16 binding element (DBE, GTAAAt/cA) of their promoters15. To test if dos-3 is a direct transcriptional target of DAF-16/FOXO, we mutated the two DBEs in the dos-3 promoter (Fig. 2a) and generated GFP transcriptional reporters using the mutated sequence. For all three transgenic lines, GFP fluorescence was lost in the PSG cells of daf-2(rf) animals (Fig. 2c), suggesting that dos-3 expression requires the binding of DAF-16/FOXO to the DBEs in its promoter and therefore it is a direct target of DAF-16/FOXO.
Disruption of dos-3 function promotes GSPC loss in daf-2(rf)
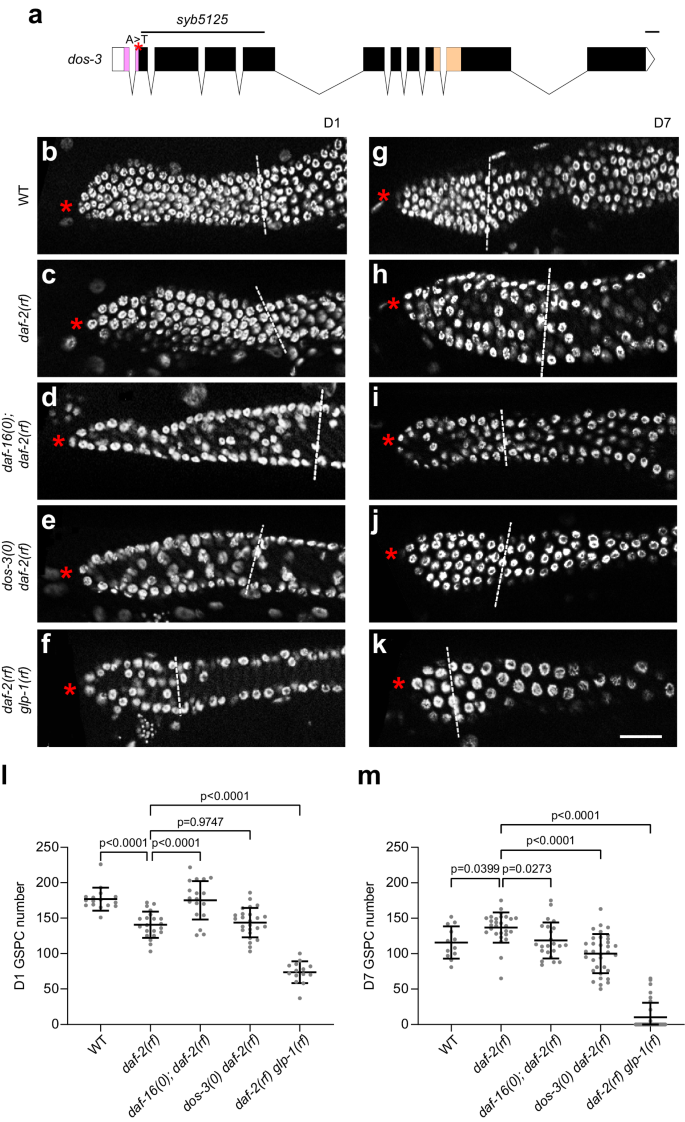
To further investigate the function of dos-3 in GSPC maintenance over time, we generated a loss-of-function allele of dos-3, syb5125, using the CRISPR/Cas9 technique. This mutation likely causes a complete loss of function of dos-3 because it deletes 864 bp after the signal peptide sequence and introduces a single-nucleotide change so that a premature stop codon forms at the deletion site (Fig. 3a).
a Schematic showing the dos-3(syb5125) mutation. The deletion and A to T single-nucleotide change in the dos-3(syb5125) allele are indicated by black line and red asterisk, respectively. Graph generated using Exon-Intron Graphic Maker (http://www.wormweb.org/exonintron). Scale bar, 100 bp. b–k DAPI-stained germ lines of D1 and D7 adult worms. Asterisk indicates the distal end of the germ line, and the white dashed line indicates the proximal border of the proliferative zone, defined as the first row of cells in which two or more crescent-shaped meiotic prophase nuclei appear. Scale bar: 20 μm. Representative germ lines of D1 wildtype (b), daf-2(rf) (c), daf-16(0); daf-2(rf) (d), dos-3(0) daf-2(rf) (e), and daf-2(rf) glp-1(rf) (f) animals. Representative germ lines of D7 wildtype (g), daf-2(rf) (h), daf-16(0); daf-2(rf) (i), dos-3(0) daf-2(rf) (j), and daf-2(rf) glp-1(rf) (k) animals. Number of GSPCs per gonad arm in D1 (l) and D7 (m) wildtype, daf-2(rf), daf-16(0); daf-2(rf), dos-3(0) daf-2(rf), and daf-2(rf) glp-1(rf) worms. Data are represented as mean ± SD with individual values shown as dots. n = 16, 21, 18, 25, 14 animals from left to right in (l); n = 12, 28, 23, 34, 32 animals in (m). P values are calculated using one-way ANOVA and Dunnett’s post hoc test. Source data are provided as a Source Data file.
In order to assess the age-associated depletion of GSPCs, we first measured the number of GSPCs at the beginning of adulthood. As expected, daf-2(rf) animals had fewer GSPCs (~140 GSPCs per gonad arm, Fig. 3c, l) than wild type (~180 GSPCs per gonad arm, Fig. 3b, l) on D1 because IIS promotes larval germline proliferation in C. elegans22. This phenotype is dependent on DAF-16/FOXO activity22, therefore loss of daf-16 reversed the number of GSPCs in daf-2(rf) mutants on D1 to a level similar to that in wild type (Fig. 3d, l). By contrast, removing dos-3 did not alter the number of GSPCs in daf-2(rf) animals on D1, suggesting that dos-3 is not required for the effect of reducing IIS on larval GSPC accumulation (Fig. 3e, l). Lastly, since C. elegans uses Notch signaling for their germline stem cell specification, e2141, a reduction-of-function mutation of glp-1, which encodes the Notch receptor expressed in the germ line, further reduced the number of GSPCs in daf-2(rf) mutants on D1 (Fig. 3f, l).
We hypothesized that in aging daf-2(rf) animals, DAF-16/FOXO activates expression of DOS-3, which is then secreted from the PSG and acts upon GLP-1 on the distal germ line to maintain GSPCs. As reported previously, we observed a delay in age-related GSPC loss in daf-2(rf) mutants9, shown by an elevation of D7 GSPC number in these animals (~130 GSPCs per gonad arm, Fig. 3h, m) compared with wild type (~110 GSPCs per gonad arm, Fig. 3g, m). Consistent with previous observations, this delay was suppressed by loss of daf-169, as evidenced by a reduction in D7 GSPC number in daf-16(0); daf-2(rf) animals even though they started with more GSPCs on D1 relative to daf-2(rf) (Fig. 3d, i, l, m). Importantly, we found that this delay was also suppressed by a mutation in either dos-3 or glp-1. Starting with similar numbers of GSPCs on D1, dos-3(0) daf-2(rf) animals maintained significantly fewer GSPCs than daf-2(rf) on D7 (Fig. 3e, j, l, m). Likewise, age-related GSPC loss was resumed in daf-2(rf) glp-1(rf) worms: these animals had a notably smaller GSPC pool than daf-2(rf) on D1, and many became GLP (all germ cells differentiated, no GSPCs remained) with age (Fig. 3f, k, l, m). Together, these results are consistent with our hypothesis that dos-3 is the main downstream effector of DAF-16/FOXO in regulating GSPC maintenance over time and that it exerts its function by impinging on the same Notch signaling pathway utilized by signals emanating from the DTC.
We used either DAPI nuclear morphology (Fig. 3) or pS/TQ antibody staining which marks early meiotic prophase nuclei23 (Supplementary Fig. 4) to determine the border of the germline proliferative zone when comparing GSPC numbers of the various genetic groups. Analysis using these two different methods led to the same conclusion: reducing DAF-2 activity delays age-associated depletion of GSPCs and this effect requires daf-16, dos-3, and glp-1 (Fig. 3l, m; Supplementary Fig. 4b, c). In addition, we performed phospho-Histone 3 (pH3) antibody staining to measure changes in the GSPC proliferation rates. Although an age-related decrease in mitotic index was detected for all genetic groups, none of the mutations seemed to cause a significant effect on mitotic index (Supplementary Fig. 4d, e). Therefore, we decided to focus on analyzing the number of GSPCs for the rest of this study.
Adult PSG dos-3 expression is required for GSPC maintenance over time
We confirmed the adult PSG requirement for dos-3(+) in GSPC maintenance over time by tissue-specific dos-3 RNAi. To achieve adult PSG-specificity, we conducted RNAi knockdown in the rde-1(0) RNAi-deficient animals expressing rde-1(+) from the PSG-specific fos-1a promoter24 and fed the worms with RNAi bacteria starting from D1. We found that similar to the case of daf-16, knocking down dos-3 only in the adult PSG resembled knocking down dos-3 in the whole animal, causing more pronounced GSPC depletion in the daf-2(rf) background (Supplementary Fig. 5). This requirement for both daf-16 and dos-3 in the adult PSG is in agreement with our hypothesis that transcription factor DAF-16/FOXO activates expression of DOS-3 in the adult PSG cells to antagonize age-related GSPC loss.
DOS-3 acts upon Notch signaling in the distal germ line
C. elegans relies on Notch signaling for germline stem cell specification. The DTC expresses two DSL ligands LAG-2 and APX-1, which interact with GLP-1/Notch receptor on germ cells. Following GLP-1 activation, its intracellular domain gets cleaved and translocates into the nucleus, where it interacts with DNA-binding protein LAG-1 to activate the transcription of downstream Notch effector genes. Two direct transcriptional targets of GLP-1, sygl-1 and lst-1, are redundantly required and each sufficient for promoting the undifferentiated, proliferation-competent germline stem cell fate25,26,27,28,29,30. Previous studies have reported that an age-related decrease in GLP-1/Notch signaling leads to changes in germline stem cell fate specification, contributing to age-related declines in meiotic entry and reproductive output11. Conversely, ectopic expression of sygl-1 could delay reproductive aging and age-related changes in germ cells31.
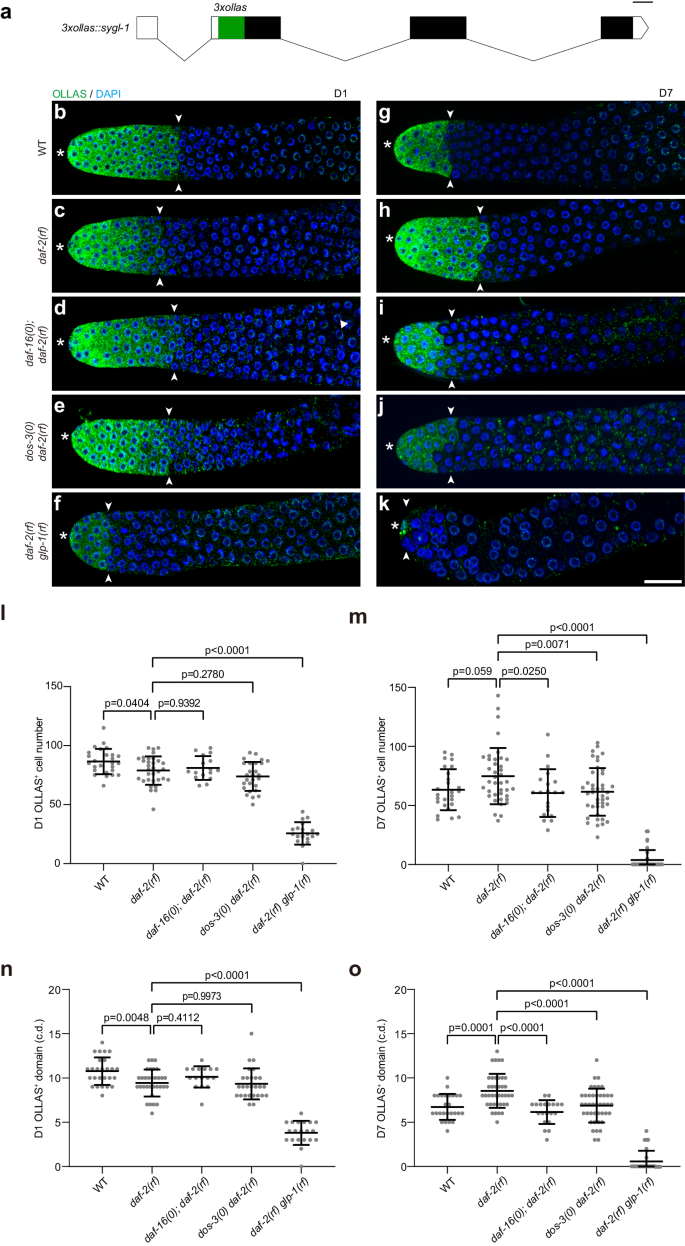
To test if DOS-3 exerts its function in GSPC maintenance over time through the previously described Notch signaling pathway in the distal germ line, we examined germline expression of sygl-1 and lst-1 in various genetic backgrounds during aging. To visualize SYGL-1 protein, we inserted a 3xOLLAS-encoding sequence into the endogenous sygl-1 locus using the CRISPR/Cas9 technique to generate an OLLAS::SYGL-1 fusion protein29 (Fig. 4a). We found that consistent with the age-associated depletion of GSPCs, wildtype animals also displayed an age-related decline in the extent of Notch signaling in the distal germ line as measured by both the number of OLLAS::SYGL-1-expressing cells (Fig. 4b, g, l, m) and the size of OLLAS::SYGL-1-positive domain in cell diameters (Fig. 4b, g, n, o). This result is in line with the observation made by Kocsisova et al. in mated wildtype hermaphrodites11, suggesting that reduction in Notch signaling contributes to age-related loss of GSPCs regardless of whether the worms are mated or not.
a Schematic showing the CRISPR/Cas9 knock-in of 3xollas at the N-terminus of sygl-1 endogenous locus. Graph generated using Exon-Intron Graphic Maker (http://www.wormweb.org/exonintron). Scale bar, 100 bp. b–k Dissected gonads from D1 and D7 adults stained with anti-OLLAS antibody (green) and DAPI (blue). Asterisk indicates the distal end of the germ line, and the white arrowheads indicate the proximal border of the OLLAS staining. Scale bar: 20 μm. Representative germ lines of D1 wildtype (b), daf-2(rf) (c), daf-16(0); daf-2(rf) (d), dos-3(0) daf-2(rf) (e), and daf-2(rf) glp-1(rf) (f) animals expressing OLLAS::SYGL-1. Representative germ lines of D7 wildtype (g), daf-2(rf) (h), daf-16(0); daf-2(rf) (i), dos-3(0) daf-2(rf) (j), and daf-2(rf) glp-1(rf) (k) animals expressing OLLAS::SYGL-1. Number of OLLAS+ cells per gonad arm in D1 (l) and D7 (m) wildtype, daf-2(rf), daf-16(0); daf-2(rf), dos-3(0) daf-2(rf), and daf-2(rf) glp-1(rf) worms expressing OLLAS::SYGL-1. Size of OLLAS+ domain measured in cell diameters (c.d.) in D1 (n) and D7 (o) wildtype, daf-2(rf), daf-16(0); daf-2(rf), dos-3(0) daf-2(rf), and daf-2(rf) glp-1(rf) worms expressing OLLAS::SYGL-1. Data are represented as mean ± SD with individual values shown as dots. n = 26, 32, 16, 27, 20 animals from left to right in (l, n); n = 26, 39, 20, 42, 33 animals in (m, o). P values are calculated using one-way ANOVA and Dunnett’s post hoc test. Source data are provided as a Source Data file.
Consistent with changes in their GSPC numbers, the age-related decrease in Notch signaling is greatly attenuated in daf-2(rf) worms: even though they started adulthood with fewer OLLAS::SYGL-1-expressing cells and a smaller OLLAS::SYGL-1-postive domain (Fig. 4b, c, l, n), these worms retained more OLLAS::SYGL-1-expressing cells and a larger OLLAS::SYGL-1-positive domain on D7 compared with wild type (Fig. 4g, h, m, o). This result suggests that aging daf-2(rf) animals maintain more GSPCs by sustaining a greater extent of Notch signaling in the distal germ line.
To test whether the attenuation of age-related decline in Notch signaling in daf-2(rf) is dependent on daf-16, dos-3, or glp-1, we examined OLLAS::SYGL-1 expression in young and aging daf-16(0); daf-2(rf), dos-3(0) daf-2(rf), and daf-2(rf) glp-1(rf) worms. We found that although removing daf-16 or dos-3 had no effect on the extent of Notch signaling in the distal germ line of daf-2(rf) animals at the beginning of adulthood (Fig. 4c–e, l, n), both significantly reduced the number of OLLAS::SYGL-1-expressing cells and the size of OLLAS::SYGL-1-positive domain on D7 (Fig. 4h–j, m, o). Consistent with being a direct transcriptional target of GLP-1/Notch, OLLAS::SYGL-1 expression was dramatically decreased in daf-2(rf) glp-1(rf) worms compared with daf-2(rf) at both time points (Fig. 4c, f, h, k). Notably, the extent of Notch signaling in the distal germ line of daf-2(rf) glp-1(rf) animals was further reduced on D7 relative to D1 (Fig. 4f, k), suggesting that the attenuation of age-related decline in Notch signaling caused by reducing IIS also requires glp-1.
We examined germline expression of lst-1 by generating a LST-1::OLLAS fusion protein29 (Supplementary Fig. 6a), and found that changes in sygl-1 and lst-1 expression in the various genetic backgrounds during aging show excellent agreement (Supplementary Fig. 6). Together, these data correlate well with our GSPC counts shown in Fig. 3, and are consistent with our hypothesis that DOS-3 mediates the effect of reducing IIS on GSPC maintenance over time by activating Notch signaling in the distal germ line.
dos-3 overexpression rescues age-related GSPC loss
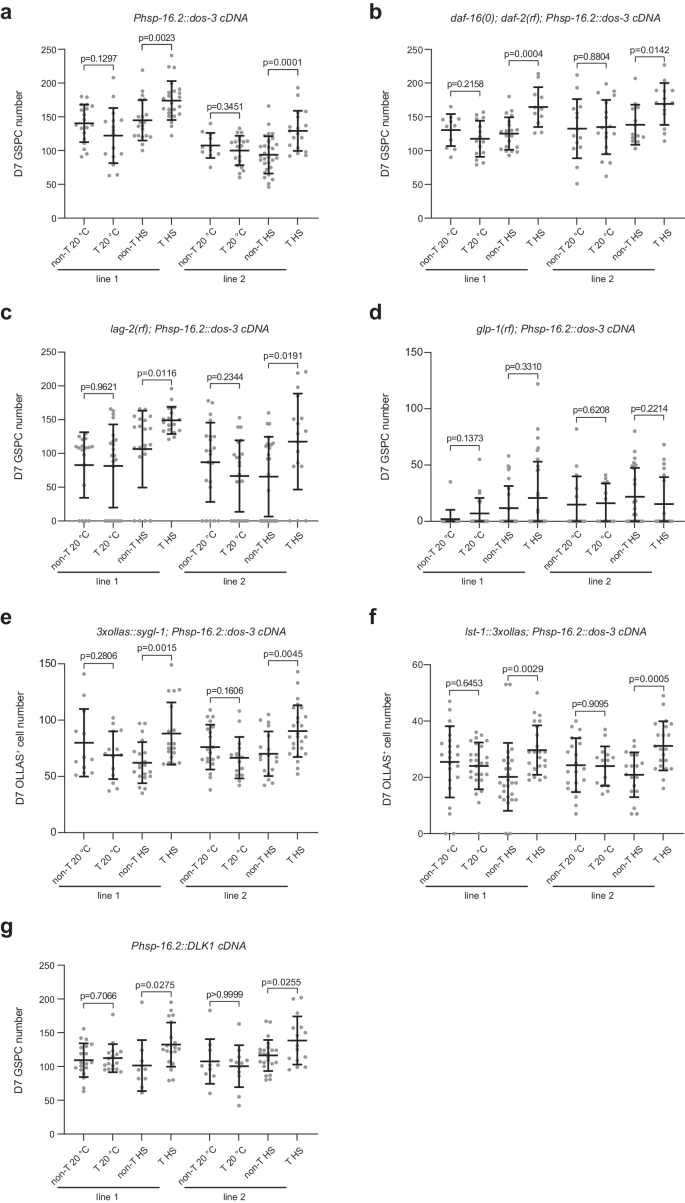
Since dos-3 is required in daf-2(rf) animals for better maintenance of GSPCs, we went on to test if overexpressing dos-3 could alleviate age-related GSPC loss in wild type. We generated extrachromosomal arrays expressing dos-3 cDNA under the control of a heat shock promoter, Phsp-16.2. Worms with the transgene and their non-transgenic siblings were either maintained at 20 °C or subjected to daily 1-h heat shock at 35 °C from D1 to D6. We found that heat shock induction of both transgenes was able to increase the number of D7 GSPCs (Fig. 5a). Therefore, we conclude that dos-3 overexpression is sufficient to improve GSPC maintenance with age.
a Number of GSPCs per gonad arm in D7 Ex(Phsp-16.2::dos-3 cDNA) worms and their non-transgenic siblings with or without daily heat shock treatment. b Number of GSPCs per gonad arm in D7 daf-16(0); daf-2(rf); Ex(Phsp-16.2::dos-3 cDNA) worms and their non-transgenic siblings with or without daily heat shock treatment. c Number of GSPCs per gonad arm in D7 lag-2(rf); Ex(Phsp-16.2::dos-3 cDNA) worms and their non-transgenic siblings with or without daily heat shock treatment. d Number of GSPCs per gonad arm in D7 glp-1(rf); Ex(Phsp-16.2::dos-3 cDNA) worms and their non-transgenic siblings with or without daily heat shock treatment. e Number of OLLAS+ cells per gonad arm in D7 Ex(Phsp-16.2::dos-3 cDNA) worms and their non-transgenic siblings expressing OLLAS::SYGL-1 with or without daily heat shock treatment. f Number of OLLAS+ cells per gonad arm in D7 Ex(Phsp-16.2::dos-3 cDNA) worms and their non-transgenic siblings expressing LST-1::OLLAS with or without daily heat shock treatment. g Number of GSPCs per gonad arm in D7 Ex(Phsp-16.2::DLK1 cDNA) worms and their non-transgenic siblings with or without daily heat shock treatment. Two transgenic lines were examined for all experiments. non-T non-transgenic, T transgenic, HS heat shock. Data are represented as mean ± SD with individual values shown as dots. n = 19, 16, 20, 23, 11, 20, 29, 18 animals from left to right in (a); n = 11, 15, 20, 11, 14, 18, 13, 14 animals in (b); n = 18, 22, 22, 17, 22, 22, 25, 17 animals in (c); n = 18, 23, 26, 32, 15, 12, 24, 20 animals in (d); n = 12, 14, 20, 18, 20, 15, 18, 25 animals in (e); n = 23, 26, 27, 23, 21, 15, 18, 21 animals in (f); n = 19, 17, 10, 20, 10, 12, 23, 15 animals in (g). P values are calculated using two-tailed Student’s t-test. Source data are provided as a Source Data file.
Our hypothesis predicts that dos-3 overexpression would compensate for loss of daf-16. We therefore tested whether ectopic dos-3 expression could rescue age-related GSPC loss in the daf-16(0); daf-2(rf) mutant. Indeed, heat shock induction of the same dos-3-expressing transgenes increased D7 GSPC counts of daf-16(0); daf-2(rf) animals (Fig. 5b), consistent with dos-3 being downstream of daf-16 in the pathway.
Based on the existing literature, it is unclear if the DOS-only proteins act as proper Notch ligands by themselves or if they function as co-ligands that facilitate the action of canonical DSL family Notch ligands. To differentiate these two possibilities, we examined the dependence of the effect of dos-3 overexpression on the presence of lag-2, which encodes one of the two DSL ligands expressed by the DTC. We found that dos-3 overexpression rescued age-associated loss of GSPCs even in a lag-2(rf) background, lag-2(q420) (Fig. 5c). On the contrary, dos-3 overexpression was not able to improve GSPC maintenance over time in the glp-1(rf) background, confirming that dos-3 acts via glp-1 (Fig. 5d). Lastly, ectopic dos-3 expression alone elevated distal germline expression of both Notch targets, sygl-1 and lst-1, on D7 (Fig. 5e, f). Together, these data suggest that DOS-3 could function as a proper Notch ligand by itself, which acts upon GLP-1/Notch receptor and activates downstream Notch targets in the distal germ line.
Human DLK1 can substitute for DOS-3
To test if this function of a C. elegans non-canonical Notch ligand is conserved across species, we repeated the above rescue experiment using a human DOS protein, DLK1. Indeed, we found that expression of a soluble isoform of DLK132 could functionally substitute for DOS-3 in vivo in C. elegans, increasing the number of GSPCs on D7 in wildtype worms (Fig. 5g). This result is consistent with DOS proteins exerting their functions by activating Notch signaling and suggests that the underlying molecular mechanism may be conserved during evolution.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41467-024-49318-6