Experimental animals
Male Sprague–Dawley rats were used in this study, and their body weights were between 250 and 300 g. The study protocols for animal procedures follow the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (2011 revision). The ethics committee of Chiba University approved the study protocol, and this study is reported in accordance with ARRIVE guidelines (PLoS Bio 8(6), e1000412,2010).
PRP preparation
This study used allograft blood instead of autograft blood14. Eight-week-old male Sprague–Dawley rats were injected intraperitoneally with anesthetics (medetomidine hydrochloride [0.06 mg], midazolam [0.8 mg], butorphanol tartrate [1.0 mg], and saline [0.58 ml]) to produce sedation and analgesia. After anesthesia, approximately 15 mL of fresh blood was collected transcardially with a syringe containing 2 mL of acid-citrate-dextrose solution A (Terumo, Tokyo, Japan) to prevent clotting. The collected blood was centrifuged (KN70; Kubota, Tokyo, Japan) at 1500 rpm for 10 min, separating the plasma fraction from erythrocytes, which was further centrifuged at 3000 rpm for 10 min to pellet platelets, as per previous investigations15. PRP necessitates activation before its use by introducing a calcium chloride solution (1 mEq/mL; Otsuka Pharmaceutical, Tokyo, Japan) and a thrombin solution (Mochida Pharmaceutical, Tokyo, Japan), each of which was one-tenth of the PRP amount. The platelet counts in fresh PRP and whole blood was determined using a hematology analyzer.
Bone marrow fluid assessment
Bone marrow fluid was obtained through autologous transplantation. The iliac bone was exposed during surgery, and an 18 G needle was used for puncturing to aid bone marrow fluid extraction. May-Giemsa staining was performed on all five blood samples to confirm the presence of bone marrow fluid (Fig. 1a). First, samples were smeared, dried, fixed with methanol, and allowed to dry naturally. Second, the cells were stained with Giemsa staining solution (Muto Corporation, Tokyo, Japan) for 1 h, washed, dried, and observed under a fluorescence microscope (BZ-X800, Keyence Corp.). Moreover, to characterize the hematopoietic progenitor population, three blood samples were harvested from bone marrow and peripheral blood and treated with ammonium chloride for 20 min to lyse the red blood cells. Then, the cells were centrifuged and stained with CD34-PE (Abcam, cat:ab223930). After 30 min of incubation on ice, the cells were resuspended with PBS + 2% FBS + 0.1% Propidium Iodide. Then, the cells were analyzed with BD FACS CantoII (BD Bioscience).
(A) Bone marrow fluid collection. Approach the iliac bone through the same skin incision as the bone graft and collect with an 18G needle and syringe. (B) Demineralized bone matrix. Fibrous structure; 0.5 ml was used for each rat. (C) The 3rd/4th/5th posterolateral lumbar fusion (PLF) surgery.
Lumbar posterolateral fusion model
Spinal surgery was performed on 20 eight-week-old male Sprague–Dawley rats10,13. The 20 rats were divided into groups of five based on the graft material used: control group (C group), artificial bone group (D group), artificial bone with PRP group (DP group), aritificial bone with PRP, and bone marrow fluid group (DPB group). Group C was only exposed at L3-5 and did not undergo bone grafting. A DBM, fibrous Grafton® (Medtronic, Tokyo, Japan), was used as the artificial bone graft substitute (Fig. 1b). The DP group received a mixture of Grafton (0.5 mL) and gel-activated PRP (0.5 mL gel-activated PRP). In the DPB group, 0.5 mL of Grafton, 0.5 mL of gel-activated PRP, and 0.2 ml of bone marrow fluid were mixed and transplanted. No corticotomy was performed in any of the groups. Each rat’s bilateral posterolateral lumbar spine was exposed through a midline skin incision followed by two paramedian fascial incisions using blunt dissection to expose the bilateral lamina and transverse processes of L3–L5 (Fig. 1c). The transplantation of graft materials to the Sprague–Dawley rats was performed by a skilled spine surgeon. Equal amounts of graft material were transplanted on both sides of the L3-5.
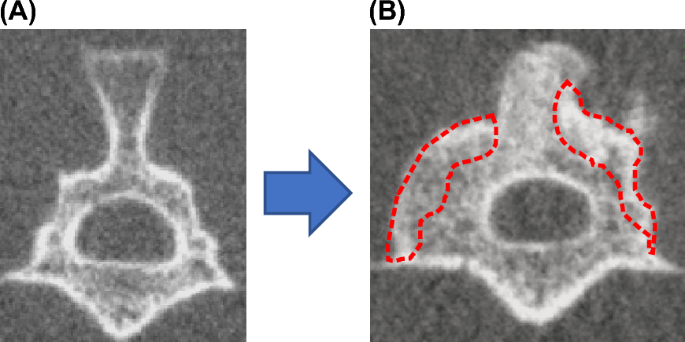
Radiographic examination (evaluation of bone union)
Computed tomography (CT) (in vivo micro-CT system, R_mCT2, Rigaku Co.) imaging was performed under inhalation anesthesia with isoflurane (1.5% isoflurane; Mylan) at 2, 4, 6, and 8 weeks after surgery. A CT image of a coronal section through the center of the L4 transverse process of the lumbar vertebra was used to measure and record the new bone formation area compared with the preoperative image (Fig. 2). ImageJ software (NIH) was used to measure bone formation. Bone union of the facet joints at L3-5 and the bone graft area were also evaluated. Bone union was defined as the cross-linking of the facet joints16. A total of four bilateral L3-5 facet joints were counted for the number of joints in which bony fusion was obtained, and the average number of bone union in facet joints in each group was then calculated. Two independent observers who were blinded to the experiment determined the bone union, and the union was accepted if all three observers agreed.
(A) Measurement of the volume of bone formation using Image J. (B) Determination of bone fusion by CT imaging.
Histological examination
Following euthanasia, the lumbar vertebrae were obtained, and a paraffin block was created using 10% neutral buffer formalin (0.1 M, pH 7.4) for fixation. Transverse sections, which were 2-μm-thick, were then produced and stained with hematoxylin and eosin14. Images of the transected posterolateral fusion (PLF) region were created using brightfield microscopy (BZ-X800, Keyence Corp.).
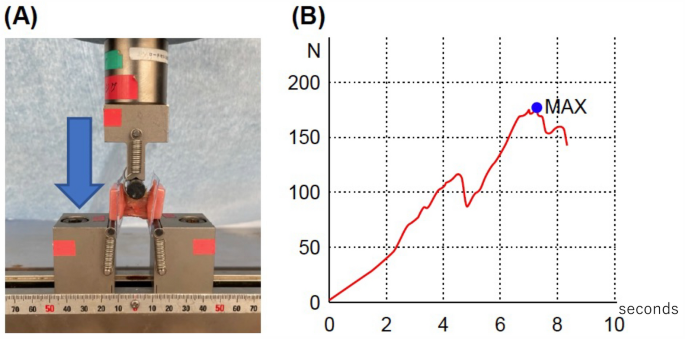
Mechanical strength examination (three-point bending test)
L3–L5 lumbar spine specimens (3.5 cm long) were excised from rats two weeks after the operation. The samples were secured on both sides with plastic holders. A three-point bending test apparatus (Shimadzu, Tokyo, Japan) was used to conduct the tests. The compression strength of the dorsal side of the spine with PLF was determined by applying force to the ventral side at two points and the dorsal side at one point. To maintain the stiffness of the specimens, they were brought to room temperature on the day of euthanization. The samples were continuously monitored while pressure was applied during the experiments. There was no occurrence of rotation or slippage of the models. A preload of 10 N of force was applied to the bones at a rate of 0.1 mm/s, and a 10-s acclimation period followed14. The mechanical strength was defined as newtons at the point of rupture, as shown in Fig. 3.
Mechanical strength examination; three-point bending test. (A) Three-point bending. (B) Representative plotting for initial peak pressure measurement.
Statical analyses
Platelet contents of plasma and PRP were compared using Student’s t-test. Average number of bone union in facet joints, bone formation, and mechanical strength among the four groups (C, D, DP, and DPB) were tested using ANOVA and Dunnett’s and Tukey’s tests as post hoc tests. The criterion for significance was set at p < 0.05.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- ChartPrime. Elevate your Trading Game with ChartPrime. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://www.nature.com/articles/s41598-023-41844-5