Human MSCs were acquired from Lonza, Inc. (Walkersville, MD, USA) and cultured at 37 °C under a 5% CO2/95% air (/v) atmosphere in MSC basal medium (Lonza, Inc.) containing MSC-GM Single Quots (Lonza, Inc.). The hMSCs at the five-passage stage were used. At 80% confluence, the cells were washed three times with phosphate-buffered saline (PBS) and replenished with serum-free DMEM (Sigma-Aldrich Corp., Tokyo, Japan) containing a 1% antibiotic–antimycotic solution (Sigma-Aldrich Corp., Tokyo, Japan). The cell-cultured, conditioned media were collected after 48 h incubation and centrifuged at 440×g and 4 °C for 5 min. The supernatants were centrifuged at 17,400×g and 4 °C for 3 min to isolate the MSC-CM. The latter were prepared immediately prior to each use. The DMEM administered to the mice was serum-free and contained the 1% antibiotic–antimycotic solution.
Histology
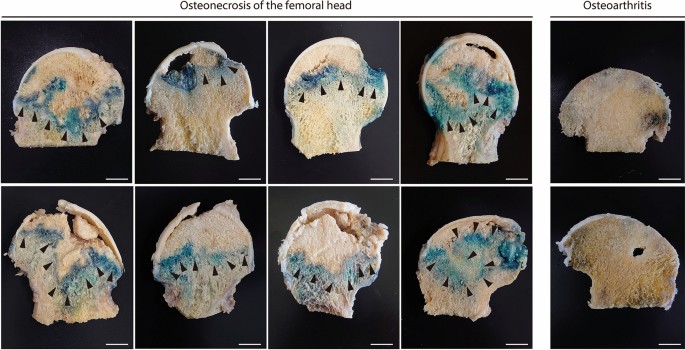
Femoral heads of humans were sliced to a width of 5 mm in the coronal plane at the median level in the axial plane. All samples were stained within 6 h of being excised. Senescence-associated β-galactosidase (β-gal) activity was measured in the femoral head tissues as previously described23. The samples were incubated at 37 °C for 24 h in β-gal staining solutions containing 1 mg/mL 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, 2 mM MgCl2, 0.01% (w/v) sodium deoxycholate, and 0.02% (w/v) Nonidet P-40. The stained femoral heads were then photographed. Samples were fixed in 4% (v/v) paraformaldehyde (PFA), decalcified with 10% (w/v) EDTA (Sigma-Aldrich Corp., Tokyo, Japan) at 4 °C for 3 weeks, embedded in paraffin, sectioned, and counterstained with Nuclear Fast Red (NFR).
Sham-operated and ischemia-operated mice were sacrificed 1 week after surgery. The distal femoral metaphysis and epiphysis were cut in half along the sagittal plane. The samples were subjected to β-gal staining as previously described. One-half of each sample was photographed at 4× magnification under a Leica M60 stereomicroscope (Leica, Wetzlar, Germany), whereas the remaining half was cut into 5-μm sections without decalcification, frozen using a cryostat (CM3050S-IV; Leica Microsystems, Wetzlar, Germany), counterstained with NFR, and photographed at 40× magnification under a BZ-X710 microscope (Keyence, Osaka, Japan).
Immunohistochemistry
Decalcified paraffin slides of the femoral head of ONFH and distal femoral epiphyses from mice at 1, 2, and 6 weeks after surgery were processed for immunohistochemistry. As primary antibodies, we used rabbit anti-phospho-histone H2A.X antibody (2577; Cell Signaling Technology, Danvers, MA, USA) and IL-6 (ab6672; Abcam, Cambridge, MA), and a goat anti-rabbit IgG antibody (Nichirei Bioscience, Tokyo, Japan) was used as the secondary antibody. Staining was performed using 3,3′-diaminobenzidine tetrahydrochloride (Nichirei Bioscience, Tokyo, Japan) for 10 min, followed by counterstaining with methyl green. Stained sections were observed and photographed at 20× or 40× magnification using a BZ-X710 microscope (Keyence, Osaka, Japan).
Immunofluorescence
A 5-mm slice of bone adjacent to the bone head section subjected to X-gal staining was decalcified with 10% (w/v) EDTA at 4 °C for 3 weeks, embedded in paraffin, sectioned, and subjected to fluorescence IF staining with p16INK4a (ab211542; Abcam, Cambridge, UK). Multiple rounds of staining were performed to disclose cellular senescence.
The necrotic, transitional, and healthy regions of the ONFH femoral head were cut into 1-cm blocks and prepared separately from the demineralized samples. Non-demineralized and unfixed 6-mm bone tissue sections were frozen and subsequently prepared using the adhesive film method developed by Kawamoto54. Briefly, the bone tissue was initially freeze-embedded in a water-soluble medium, following which an adhesive film was applied to the cut surface to support the frozen sample whilst being cut using a disposable tungsten carbide blade. Sections of 6-µm thickness were prepared using a cryostat (CM3050S-IV; Leica Microsystems, Wetzlar, Germany). Immunofluorescence staining was conducted with β-galactosidase (ab9361; Abcam), nestin (ab92391; Abcam), periostin (ab14041; Abcam), and DMP-1 (NBP1-45525; Novus Biologicals, Centennial, CO, USA). The sections were mounted with VectaShield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI). In vitro, Immunofluorescence staining was performed using β-galactosidase (ab9361; Abcam) and p16INK4a (ab211542; Abcam) and the sections were mounted with VectaShield mounting medium containing DAPI. The sections were examined at 40× magnification under a BZ-X710 fluorescence microscope (Keyence, Osaka, Japan) and the β-gal-positive cells were counted using a BZ-X 800 Analyzer (Keyence, Osaka, Japan). 315, 809, and 216 nestin + cells were observed in the healthy, transitional, and necrotic regions, respectively, as were 616, 2218, and 291 periostin + cells, and 630, 1321, and 321 DMP-1 + cells, respectively.
Multiplex staining for β-gal, nestin, periostin, and DMP-1 was performed on non-demineralized, unfixed mouse knee sections at 2 weeks after surgery, as previously described for human samples. The mouse knee sections were also subjected to β-galactosidase and TRAP staining (ab191496; Abcam). The cells were blindly counted using a BZX800 analyzer (Keyence, Osaka, Japan).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
In humans, the expression levels of p16INK4a, p21, p53, RB, p53, RANKL, IL-6, and MMP-3 were determined by using RT-qPCR to measure their mRNA levels. Bone tissue from the necrotic, transitional, and healthy regions were cut into 5-mm blocks, flash-frozen immediately after excision, and pulverized with a Multi-Beads Shocker (Yasui Kikai Co. Ltd., Osaka, Japan). In mice, the expression levels of p16INK4a, p19, p21, p53, RB, IL-6, MMP-3, BMP2, RANKL, DKK1, and sclerostin were determined by measuring their mRNA levels using RT-qPCR. At 1 week post-surgery, mice were sacrificed and their distal femoral epiphyses were immediately excised. The cartilage was dissected with a scalpel and frozen. Total RNA was extracted and isolated from all bone samples with the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The relative mRNA levels of the target genes were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). All measurements were analyzed by the 2−ΔΔC(t) method55. The primer sequences are listed in Table S1.
Micro-CT
Radiographic analysis of the distal femoral epiphysis was performed by subjecting the mice to µ-CT scan (Skyscan 1176; Bruker, Kontich, Belgium). The operating parameters were 50 kV X-ray voltage, 500 μA X-ray current, 0.5 mm Al filter, 0.5° rotation step, 9 μm pixel size, and no frame averaging. The µ-CT scan images were reconstructed with Skyscan NRecon software and analyzed by using its 3D algorithms according to the manufacturer’s instructions. To evaluate the degree of epiphyseal collapse, the epiphyseal height-to-width ratios were calculated using the measurement obtained for the coronal sections of the µ-CT images35. The region of interest was the cancellous bone at the distal femoral epiphysis surrounded by outlined cortical bone. The bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) were calculated to delineate and define the epiphyseal trabecular compartments.
Histological analysis
Bone specimens were fixed in 4% (v/v) PFA at 20 ℃ for 2 weeks, decalcified with 10% (w/v) EDTA (Sigma-Aldrich Corp.) at 4 °C for 3 weeks, and embedded in paraffin. Sagittal sections (5-µm) were cut using a Sakura IVS-400 sledge microtome (Sakura Seiki, Tokyo, Japan), subjected to hematoxylin and eosin (H&E) staining, and examined microscopically. One week after surgery, certain bone sections were subjected to TdT-mediated dUTP nick end labeling (TUNEL) staining (Fujifilm, Osaka, Japan) and counterstained with methyl green.
Histomorphometry
Double labeling was performed by subcutaneously injecting the mice with 10 mg/kg calcein at 6 d and 2 d before euthanasia. Five mice in the DMEM group and five mice in the MSC-CM group underwent bone histomorphometry 4 weeks after surgery. After sacrifice, the right knee joints of mice were resected, fixed in 70% (v/v) alcohol, stained with Villanueva bone stain, and embedded in methyl methacrylate resin (Wako Pure Chemical Industry, Osaka, Japan) without decalcification. The resulting blocks were sectioned using a Reichert-Jung microtome (model 2050; Finetech Scientific Instruments, Tokyo, Japan) along the frontal plane at 5-μm thickness. An observer from an external institution blinded to the experimental treatments and groups evaluated the number of osteoblasts per bone surface (N.Ob/BS), the number of osteoclasts per bone surface (N.Oc/BS), and the bone formation rate per bone surface (BFR/BS).
Statistical analysis
Unpaired Student’s t-test, one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test, and one-way repeated-measures ANOVA with Bonferroni’s post-hoc test were performed in SPSS v. 28 (IBM Corp., Armonk, NY, USA) and Origin 2023 (OriginLab Corp., Northampton, Massachusetts, USA). Effect size was determined by Cohen’s d method. Data are means ± standard deviation (SD). p < 0.05 was considered statistically significant.
Other materials and methods
Details of other materials and methods are available in the Supplementary Information. This section includes detailed descriptions of isolation of human osteonecrosis of the femoral head (ONFH) cells, staining of the bone marrow of the mice, and real-time quantitative polymerase chain reaction (qPCR).
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-024-53400-w
