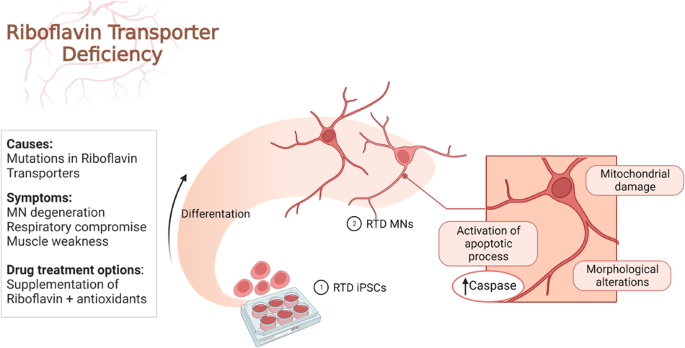
Maintenance of iPSCs
For this study, we use iPSCs derived from skin biopsies of four RTD patients as described in Magliocca et al., (submitted). The patients carry the following mutations: RTDmut Pt1 (c.155 C > T and c.1255 G > A; SLC52A2mut p. S52F; p. G419S); RTDmut Pt2 (c.155 C > T and c.935 T > C; SLC52A2mut p. S52F; p. L312P); RTDmut Pt3 (c.505 C > T and c.1030_1031del; SLC52A2mut p. Arg169Cys; p. Leu344AlafsX100); RTDmut Pt4 (c.505 C > T and c.593 G > A; SLC52A2mut p. Arg169Cys; p. Trp198Ter). As a reprogramming method, the non-integrative episomal technology (#SC301A-1, Minicircle DNA and mc-iPS Cells, Euroclone, Milan, Italy) was used. The iPSCs were plated on 6-well Matrigel-coated multiwells (BD Biosciences, San Jose, USA), kept in culture in mTeSR1 plus Basal Medium (#05826, Stem Cell Technologies, Vancouver, Canada) in an incubator at 37 °C with 5% CO2 and 21% O2. When iPSCs reached about 70% confluence, they were dissociated using EDTA (ethylenediaminetetraacetic acid) and plated on newly Matrigel-coated wells.
Differentiation of iPSCs into Motor Neurons
iPSCs were differentiated into MNs by adapting the protocol proposed by Corti et al. [32]. Cells were grown for 10 days in NeuroCult NS-A Basal Human Medium (#05750, Stem Cell Technologies), after which retinoic acid (#R2625, Sigma Aldrich, St. Louis, MI, USA) was added. The culture medium was replaced on alternate days until day 17 when, in addition to 0.1 µΜ retinoic acid, 2 µΜ dorsomorphin (#P5499, Sigma Aldrich) and 3 ng/ml activin A (#120-14E, PeproTech, Rocky Hill, CT, USA) were added. Starting from day 25, until the end of the differentiation protocol, the culture medium was replaced with BrainPhys Neuronal Medium (#05790, Stem Cell Technologies), containing 200 μM ascorbic acid (#A4403, Sigma Aldrich), 2 μg/ml GDNF (#450-10, PeproTech), 10 ng/ml BDNF (#450-02, PeproTech), SM1 (#05711, Stem Cell Technologies) and N2 (#17502-001, Thermofischer Scientific, Waltham, MA, USA).
TUNEL assay
For TUNEL assays (#G3250, Promega, Madison, WI, USA), cells were plated on slides and fixed with 4% formaldehyde solution in phosphate buffer saline (PBS) for 10 min, at room temperature (RT). Cells were permeabilized by 0.1% Triton X-100 for 10 min at 4 °C, then washed with PBS. Equilibration Buffer was added to samples for 5 min at RT, prior to incubation with 45 µl Equilibration Buffer, containing 5 µl Nucleotide Mix and 1 µl TdT, for 1 h at 37 °C. The reaction was stopped by adding 2X SSC for 15 min and nuclei were contrasted by Hoechst (1: 10000 for 10 min).
Immunofluorescence and confocal microscopy
After differentiation, RTD and healthy iPSCs and MNs were fixed with 4% formaldehyde for 10 min at RT, then incubated with a blocking and permeabilizing solution, composed of 5% Bovine Serum Albumin (BSA, #10775835001, Roche, Basilea, Switzerland); 0.1% Triton X-100 (Sigma-Aldrich) in PBS, for 1 h at RT. Cells were treated with the primary antibody of interest, diluted in PBS containing 3% BSA, as follows: 1:100 Activated Caspase 3 #C8487, Sigma Aldrich; 1:100 AIF #4642, Cell Signaling (Danvers, MA, USA); 1:500 βIII TUB #T8578, Sigma Aldrich; 1:200 Mitochondria #NBP2-32982, Novus BIO (Centennial, CO, USA), 1:100 SLC52A2 #CSB PA060150, CusaBio (Houston, TX, USA). Slides were immersed in buffer, then incubated with appropriate secondary antibodies, conjugated to either of the following, diluted 1:500 in PBS, for 1 h: Alexa Fluor 488, Alexa Fluor 555 or Alexa Fluor 647 (Invitrogen, Carlsbad, CA, USA). Nuclei were counterstained using 1:10000 Hoechst (#33342, Invitrogen) in PBS for 10 min, at RT. After mounting with 1:1 PBS/Glycerol, slides were observed in a Leica TCS SP5 (Leica Microsystems, Wetzlar, Germany) confocal microscope or Olympus FV3000 (Evident Europe GmbH, Olympus, Microsystems, Hamburg, Germany) equipped with 405 nm-488 nm-561 nm and 640 nm diode lasers. Representative images were captured and assembled using Adobe Photoshop CS6 software (Adobe Systems Inc., San Jose, CA, USA).
Western blot analyses
For western analysis, cells were lysed in buffer composed by RIPA (#S-R0278, Sigma) protease inhibitor cocktail (Roche, Basilea, Swiss) and 0.5 mM Sodium Orthovanadate. cell extracts were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (#1704159, Bio-Rad, Hercules, CA). Membranes were blocked in 5% milk for 1 h at RT and incubated with primary antibodies overnight at 4 °C. Blots were incubated with appropriate secondary antibodies (#111-035-003, Jackson ImmunoResearch, United Kingdom) for 1 h at RT and stained with SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnologies, Massachusetts, USA). The following primary antibodies were used: Act casp-31:1000 overnight (#C8487, Sigma Aldrich) and GAPDH 1:10000 overnight (#ab8245, Abcam).
Electron microscopy
For SEM analysis, cells were plated on coverslips, fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, for 45 min at 4 °C. After washing, cells were post-fixed with 1% OsO4 in the same buffer for 45 min, at 4 °C in the dark, then dehydrated by ethanol and hexamethyldisilazane (HDMSO). Air-dried slides were mounted on metal stubs by bi-adhesive carbon discs and gold-coated by Emitech K550 Sputter Coater. Electron micrographs were acquired by a Gemini 300 SEM (Carl Zeiss AG, Jena, Germany), detecting secondary electrons with an operating voltage of 5 kV.
For focused ion beam/scanning electron microscopic (FIB/SEM) analyses, cells were plated in chamber slides (Lab-Tek™ II Chamber Slide System, ThermoFischer) and fixed in a mixture of 2% formaldehyde, 0.5% glutaraldehyde in 0.1 M cacodylate, pH 7.4, for 45 min, at 4 °C. After washing and post-fixation performed as above, samples were contrasted with UranyLess (Electron Microscopy Science, Foster City, CA, USA), for 1 h at 4 °C. Cells were dehydrated in ethanol, and infiltrated by a mixture 1:1 of ethanol and epoxy resin (Sigma-Aldrich, Cat# 45359-1EA-F, Burlington, MA, USA), for 1 h at RT, then embedded in absolute resin. After polymerization at 60 °C for three days, samples were selectively milled using FIB column, operated at a voltage of 30 kV and a current of 9.3 nA, to expose regions of interest to be imaged. Micrographs were acquired by SEM column, detecting backscattered electrons at a working distance of 2 mm and using the Everhart-Thornley detector (ETD) with a voltage of 2 kV and a current of 0.34 nA. Images were assembled using Adobe Photoshop CS6 software (Adobe Systems Inc., San Jose, CA, USA).
Statistical analyses
LAS X software was used to acquire and quantify fluorescence images, while for immunoblotting analysis, Image J software was used to quantify band staining. Statistical analyses were performed using Prism software (GraphPad Software, Inc., La Jolla, CA, USA), followed by parametric (Student’s t test, one-way ANOVA) or non-parametric (Mann–Whitney, Kruskal-Wallis) tests, to compare sample groups. For FIB/SEM analyses, approximately 20 cells per sample were analyzed. For IF, TUNEL assay and WB, a minimum of 3 technical and 3 biological replicates were performed for all experiments. Data were expressed as mean and standard error of the mean ± SEM of n ≥ 3 independent experiments and defined as ****p ≤ 0.0001; ***p ≤ 0.001; **p ≤ 0.01; *p ≤ 0.05.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41420-024-01812-y
