Cell culture
ISP-4, C2C12, and immortalized porcine muscle satellite cell (PMSC, a kindly gift from Professor Li-Min Hou, Nanjing Agriculture University) were cultured in growth medium (GM), containing Dulbecco’s modified Eagle’s medium (Servicebio#G4511), 10% fetal bovine serum (Procell#164210-50), 100U/ml penicillin (Sangon#A610028), 100 µg/ml streptomycin (Sangon#A610494). For PMSC, an extra 10% FBS and 2 ng/mL FGFb (Novoprotein#C046) were added.
ISP-4 adipogenic differentiation medium (ADM) consisted of growth medium supplemented with 1 µg/mL Insulin (Novolin R), 5 nM 3,3’,5-Triiodo-l-thyronine (Yuanye Bio-Technology#S24025-25mg), 2 µg/ml dexamethasone (Sangon#A601187), 100 µM 3-isobutyl-1-methylxanthine (IBMX, Sangon#A606630), 125 µM indomethacin (Makclin#I811784), 1 µM rosiglitazone (Makclin#R832516), 33 µM biotin (Makclin#B6220), 17 µM Pantothenic acid (Sangon#A600683), 1 µg/µl Transferrin (Yuanye Bio-Technology #S12027). ISP-4 adipogenic maintenance medium (AMM) includes growth medium supplemented with 1 µg/mL Insulin (Novolin R).
For the 4 + 4 protocol, the cells were first recovered in a growth medium for 2 days, followed by a switch to ADM for 4 days. Afterward, the cells were maintained in AMM for an additional four days, mediums were changed every 2 days.
For the 2 × 5 protocol, the cells were seeded and cultured in AMM for 10 days, with medium changes every 2 days.
Cell culture in alginate hydrogel
To prepare the cell/alginate suspension, ISP-4 cells were resuspended in a 0.75% alginate solution (Sigma#W201502) at a concentration of 1.25 × 107 cells/ml. The suspension was then injected into a solidification buffer (50 mM CaCl2) and placed in a 37 °C incubator for 20 minutes to solidify into microfibers. After washed with DMEM, the microfibers were transferred into a growth medium (GM) for recovery before adipogenesis according to different protocols.
Cell culture in wire-drawing protein scaffold
Irradiated sterilized wire-drawing peanut protein was soaked in growth medium overnight and then cut into small cubes (about 1 cm2 in area and 0.3 cm in thickness). These cubes were subsequently dried using sterile filter paper and placed onto a 10 cm dish for later use.
To seed cells onto the scaffolds, 2.5 × 106 ISP-4 were resuspended in 7 µl thrombin (20NIH units per ml, Biosharp#BS903). Next, 7 µl fibrinogen (15 mg/ml, Biosharp#BS943) was added immediately before seeding the cells onto the scaffolds. The cell blend was mixed well and dropped onto the scaffolds. The dish with seeded scaffolds was then placed into a 37 °C incubator for 20 minutes to allow the fibrin to gel. Once gelation had occurred, a growth medium was added to the dish, and the scaffold with cells was cultured as indicated.
RNA extraction, cDNA synthesis, and RT-qPCR
RNA isolation was performed using Total RNA Extraction Reagent (Vazyme#R401), followed by reverse transcription using HiScript II Q RT SuperMix (Vazyme#R223), as per the manufacturer’s guidelines. Real-time quantitative PCR (RT-qPCR) was carried out with iQ SYBR Green Supermix (Servicebio#G3326) using the QuantStudio™ 5 System (ThermoFisherScientific). The sequence of primers is listed in supplementary Table 1. The 2−∆∆ct method was used to calculate relative gene expression, with 36B4 as the reference gene for normalization.
Fluorescence staining
For 2D cultured ISP-4 or mixed culture cells of ISP-4 and C2C12/PMSC, samples were fixed in 4% paraformaldehyde. After washing with PBS, fixed cells were permeabilized with 0.2% Triton X-100, then blocked with 3% BSA solution, anti-DESMIN (Abclonal, Cat#A0699, Lot#5500004718) and anti-MYL1 (Servicebio, Cat#GB112474, Lot#AC230928017) were used to detect mature muscle cells. After being washed with PBS, cells were re-stained within Goat Anti-Rabbit IgG-DyLight 549 (Bioworld, Cat#BS10023, Lot#CL89330), Hoechst33342 (1:1000, Beyotime#C1028), and BODIPY FL (1:5000, Thermo Fisher#D3922).
For alginate hydrogel, samples were fixed in 4% paraformaldehyde containing 50 mM CaCl2. The samples were stained with Hoechst33342 (1:1000, Beyotime#C1028), BODIPY FL (1:5000, Thermo Fisher#D3922) and Actin-Tracker Red-Rhodamine (1:200, Beyotime#C2207S) in PBS for 30 min. And the samples were washed with PBS before imaging.
ISP-4 and C2C12/PMSC mixed culture
For coculture with C2C12, a suspension of ISP-4 cells (1 × 105 cells) and C2C12 cells (3 × 105 cells) was seeded in six-well plates containing growth medium. The cells were then cultured for 48 h. Subsequently, differentiation was initiated by switching to ADM, and after 4 days, the medium was exchanged into AMM for another 4 days. Then the medium was changed into AMM with 2% horse serum to initiate myogenesis.
For coculture with PMSC, 0.6 × 105 ISP-4 cells and 1.4 × 105 PMSC cells were seeded into a 12-well plate with 1 mL culture medium. After 24 h recovery, cells were differentiated with ADM for 48 h, and AMM for another 48 h. Then cells were cultured in AMM with 2% horse serum for the other 4 days.
The cells were maintained in a 5% CO2 humidified incubator at 37 °C, with medium exchanges every 2 days.
Microcarrier cell culture
100 mg of 3D TableTrix microcarriers (CytoNiche) were dissolved in 20 mL of growth medium in a 125 mL sterile spinner flask (CytoNiche) to obtain a final concentration of 5 mg/mL. The mixture was left to dissolve overnight at 4 °C. The next day, the spinner flasks were prewarmed to 37 °C, and a total of 2.5 × 106 ISP-4 cells in 30 ml growth medium were added.
The spinner flasks were positioned on magnetic stirring platforms in the incubator. For the first 24 h, rotation speed was set to 35 rpm for 5 min, then followed by 0 rpm for 1 h, and repeated 24 times. Afterward, the rotation mode was changed to a constant speed of 40 rpm. 50% growth medium was changed every 48 h.
For cell counting, 1 mL of medium containing microcarriers was collected from a spinner flask. 200 µL of supernatant was removed and replaced with lysate (CytoNiche). After incubating at 37 °C for 30 min, the cells were then counted using a hemocytometer,
To assess the differentiated microcarriers in culture flasks, 1 mL of cell suspension was pipetted and stained with Hoechst33342 (1:1000, Beyotime, C1028) and BODIPY FL (1:5000, Thermo Fisher, D3922).
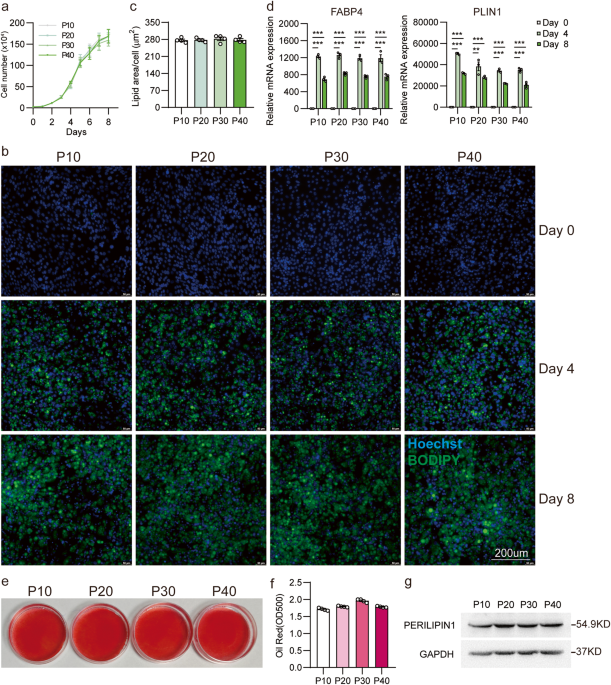
Area of lipid droplet analysis
All images were taken by confocal laser scanning microscope (LSM750, Zeiss) under the same acquisition conditions. Images were processed in ImageJ (version: 2.1.0/1.53c) as the following step. 1. Scale bar calibration: Open the image with only BODIPY staining, measure the length of the image’s scale bar, and set the pixel-to-length (µm) ratio in “set scale”. 2. Fluorescence area measurement: Convert the image to “8-bit”, open “Threshold”, adjust the threshold, select the green fluorescent area, and select “Measure” to obtain the area of the lipid droplets. 3. Cell nuclei counting: open the image with only nucleus staining, and use ImageJ’s “analyze particles” tool to count the cell nuclei stained with Hoechst.
Oil-red staining
Cells were fixed with 4% PFA at 4 °C overnight. Following fixation, the cells were rinsed with 60% isopropanol and then stained with Oil Red O (0.42 g/mL in 60% isopropanol) for 5 min. The Oil Red O solution was subsequently removed, and the cells were washed three times with tap water. The Oil Red O stain was then extracted using isopropanol and the absorbance was measured at 500 nm.
Western blot
Protein was extracted with RIPA (Servicebio#G2002), target proteins were detected with antibodies as follow: anti-PERILIPIN 1 (Bioss, Cat#bs-3789R, Lot#AD121123), anti-DESMIN (Abclonal, Cat#A0699, Lot#5500004718), Anti-FLAG (Genscript, Cat#A00187, Lot#155000952), Anti-GAPDH (Bioworld, Cat#AP0063, Lot#AA55151), anti-TUBULIN (Sangon, Cat#D190090-0100, Lot#H508AA0104).
Lipid content analysis
The lipid content was measured using a triglyceride content assay kit (Sangon#D799795), based on colorimetric method, following the manufacturer’s instructions. For sample preparation, ~0.1 g of hydrogel or microcarrier was homogenized using a blue pestle in 1 ml Buffer 1. Then, 200 µL of supernatant was collected for further analysis. 200 µL solution with 1 mg/mL triglyceride was used for comparison. The lipid content was calculated using the following formula.
$${rm {L{ipid}}},{rm {{content}}}= left(1,{rm {{mg}}}/{rm {{mL}}}times 0.2,{rm {{mL}}}right) times frac{{A}^{{rm {{sample}}}}-{A}^{{rm {{Blank}}}}}{{A}^{{{rm {stander}}}}-{A}^{{{rm {Blank}}}}}/,{{rm {sample}}; {rm {weight}}},({rm {g}})$$
Statistics and reproducibility
Statistical analysis was performed using Prism 9.0 (GraphPad). Analysis between two groups was performed using a Two-tailed unpaired Student’s t-test. Sample size has been indicated in each figure legend.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s42003-023-05583-7
