ADSCs are adult stem cells with great potential to differentiate into multilineage cells in vivo as well as in vitro. Our previous studies have shown the functional benefit of ADSC injection in erectile dysfunction models1,10. intracavernous injection is a locally applied intervention that is commonly thought to target the corpus cavernosum directly11. The penis is in fact a vascular organ, and intracavernous injection is similar to intravenous (IV) injection. It has been demonstrated that IV injection of ADSCs can restore erectile function in a rat model of radiation therapy-induced ED10. Lue et al.12,13 demonstrated that the majority of intracavernous injected stem cells exited the penis within 1 day, and preferentially traveled to the bone marrow or to the major pelvic ganglion in rats with cavernous nerve injury. It has been increasingly observed that the transplanted SCs did not necessarily engraft and differentiate at the site of injury but might exert their therapeutic effects through secreted trophic signals14.
However, the post-transplant complications commonly due to improper cell homing and engraftment. Therefore, tracing transplanted cells in vivo to understand the fate of these cells is necessary for systematic investigation of cell therapy. Unfortunately, none of most researchers’ attempts to find the transplanted cells in penile tissues has panned out, even though the animals clearly demonstrated functional and structural improvements. To monitor the distribution and survival of transplanted stem cells in penile tissues, most previous studies have used cells labeled with LacZ, DAPI, GFP, DiI, BrdU, or EdU11. Moreover, rather than allowing non-invasive in vivo imaging to trace transplanted cells in real time, animals in studies using these labeling methods had to be killed for histological examination.
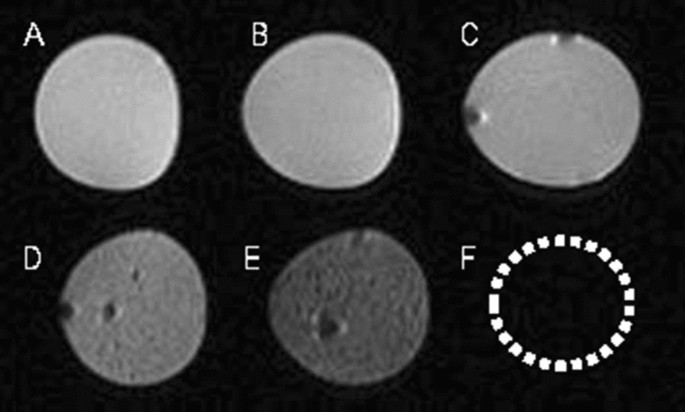
In recent years, many studies have reported the feasibility of non-invasively monitoring the transplanted cells by MR imaging in vivo15,16,17. Such cell tracing method makes the transplanted cells directly visible for us, which is important for the development of cell therapy. In our current study, we found that ADSCs labeled with SPIONs could be detected in the corpus cavernosum with MR imaging; MR images of SPION-labeled ADSCs showed a strong hypointense signal compared with control groups, which included distilled water, unlabeled ADSCs and agarose gel. We also carried out in vitro MR images of SPION-labeled ADSCs at different concentrations to assess the sensitivity of the MR imaging. A clear hypointense signal at all concentrations was detectable, but the cells forming a cluster of 1 × 106 or more showed the greatest hypointense signal on T2-weighted MR imaging, which indicated the optimal dose for in vivo studies. We therefore decided to use 1 × 106 SPION-labeled ADSCs for in vivo studies monitoring their distribution in penile tissues. In this study, we confirmed MR imaging in vivo made us non-invasively monitor the labeled ADSCs in normal rat and pig corpus cavernosum.
It has been demonstrated that ADSCs lack major histocompatibility complex-II expression, and can control graft-versus-host disease18. Both preclinical and clinical studies have shown that allogeneic transplantation of ADSCs offers no obvious advantage over xenotransplantation19. In this study, we observed no evidence of immunological rejection, and confirmed that even xenogeneic ADSC transplantation was safe in vivo. The theoretical basis of our study was that SPION-labeled ADSCs showed a clear hypointense signal on T2*-weighted MR imaging, and the T2*-weighted sequence was more sensitive for the detection of iron than T2 weighted sequence. On follow-up studies, the hypointense signal intensity faded over time, and SPION-labeled ADSCs could be tracked over the course of 1 week after transplantation. Further, retention of ADSCs in rat corpus cavernosum was assessed by Prussian blue staining. Histopathology confirmed engraftment of labeled ADSCs, with slow dilution of the iron label over time. Mention that most studies based on an experimental rat model, we injected labeled cells into pig corpus cavernosum to simulate the fate of injected cells in clinical trials. The T2*-weighted signal intensity in pigs increased over time, similar to the MR imaging results in rats.
The data from this study showed that MR imaging can be used as a new approach to track ADSCs at least in the short term in rat and miniature pig corpus cavernosum. Despite all this, we still believe MR imaging is a promising approach to trace stem cells even in long term in the context of erectile dysfunction20. In condition of cavernous nerves injury models, several preclinical trials attempted to improve stem cells implantation by combining them with scaffolds (PLGA membrane, hydrogel or Matrixen)21 or cellular self-assembling into micro-tissues22. We speculate that MR imaging will fully demonstrate its advantages in tracking SCs under these condition23,24,25. However, Song et al.9 observed that the majority of injected cells remained at or near the injection site even 12 weeks after injection. The use of MR imaging to monitor injected cells in the corpus cavernosum is our preliminary study, which should be investigated further. MR imaging had several advantages for elucidating the fate of transplanted cells over other imaging modalities, such as the ability to image transplanted cells longitudinally at high spatial resolution without exposure to ionizing radiation, and the possibility to co-register anatomical structures with molecular processes and functional changes26. However, since MR imaging was still in its infancy, it currently faced a number of challenges. one major disadvantage was that the label itself was detected rather than the cells of interest26.
It was interesting to note that at day 1 after ADSC injection the corpus cavernosum showed a marked, hypointense T2*-weighted signal. This possibly resulted from the retention of the majority of labeled cells in the corpus cavernosum, as reported by Lue et al.8,9. Then the MR signal intensity faded over time, associated with the negative Prussian blue staining. Considered that the stability of SPION-labeled-ADSC should be checked until 28 days, it often needed to exclude the possibility that ADSCs were present due to natural decomposition but were not observable because they had been unlabeled. For ADSCs cultured in vitro, SPIONs would be excreted along with cell differentiation or via extracellular vesicles (EVs). Therefore, we did not investigate the stability of SPION-labeled ADSCs, and we boldly speculated that SPIONs would be mostly depleted in ADSCs cultured for 28 days. Of note, surface modification techniques for SPIONs had been proven to enhance stability of SPION-labeled-stem cells and improve the therapeutic effects of stem cells27,28. After injecting stem cells into the corpus cavernosum of the penis, less than 1% of the stem cells were able to remain in the corpus cavernosum, and this percentage continued to decrease over time. By the fourth week, the number of detectable SCs was extremely limited29. The majority of SCs belonged to the bone marrow30. Increasing evidence suggested that SCs did not necessarily need to differentiate into specific cell lineages in the injured area, but rather promote angiogenesis, inhibit apoptosis, or cell death, and regulate the immune system through paracrine or autocrine secretion of a series of cell-nourishing factors14.
This was our preliminary exploratory research, aimed at providing researchers with an option: MR imaging could be used in stem cell therapy to monitor the engraftment of stem cells more intuitively in local tissues, especially when using materials such as collagen or hydrogels to promote local engraftment of stem cells31. In vivo, SPIONs (a type of conventional MR imaging contrast agent) was typically taken up by phagocytic cells (macrophages and Kupffer cells), then cleared by the liver and spleen, and ultimately excreted in bile. Furthermore, whether SPIONs were involved in the iron metabolism pathways of cells and whether they affect the biological function of ADSCs would be further studied in our subsequent research. However, in our preliminary research, we had already explored the effective concentration of SPIONs labeled on ADSCs, and found that it had little effect on the proliferative activity of ADSCs. Moreover, SPION-labeled ADSCs could migrate and engraft under the influence of an external magnetic field in vitro32.
Due to technical issues such as injections into the subcutaneous tissue or urethra, or leakage of cell suspension, some of the MRI results in rats might not accurately reflect the engraftment status of ADSCs after corpus cavernosum injection. Therefore, we showed the representative photos. On the other hand, we were unable to perform quantitative analysis of the signal intensity for the specified region in MRI imaging, nor could we conduct quantitative analysis of the area for the designated region. Our experimental results visually demonstrated the engraftment of ADSCs after injection into the corpus cavernosum of rats, and we guaranteed the reproducibility of this experiment. In addition, in our previous research, we had confirmed that magnetic targeting SPIONs-labeled ADSCs could promote the engraftment of ADSCs in the corpus cavernosum and improve erectile function in diabetic rats. This study was a supplement to our previous research, aiming to explore the feasibility of using MR imaging for real-time monitoring SPION-labeled- ADSCs in the corpus cavernosum. However, considering the time and cost constraints associated with the 7.0 T MRI examination, we were unable to repeat the experiments using the ED rat model.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-023-51076-2
