Isolation and purification of biosource squalene and its derivatives
About 323 mg of the desired compound with 99% purity was recovered from 100 g of leaf dust of Cocos nucifera L. Thin Layer Chromatographic (TLC) run of pure squalene standard (Sigma-Aldrich), bio-source squalene extracted from Artocarpus lakoocha leaves19 (BSQ) and purified pentane extract of coconut leaves, exhibited spots with same Rf value (6.8) under iodine vapour. So, it indicated that the compound present in these extracts would be a more or less similar category.
Identification of 4,4′-diapophytofluene through spectral analysis
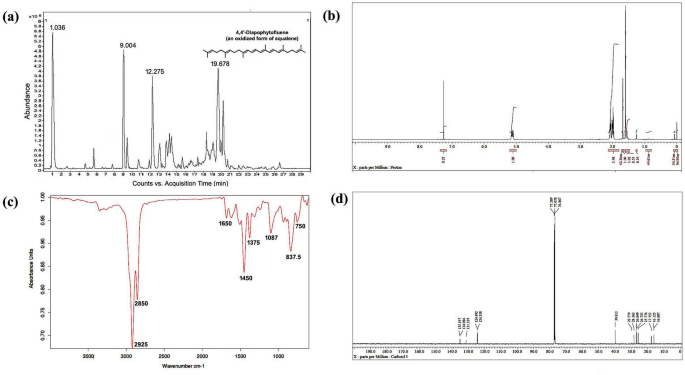
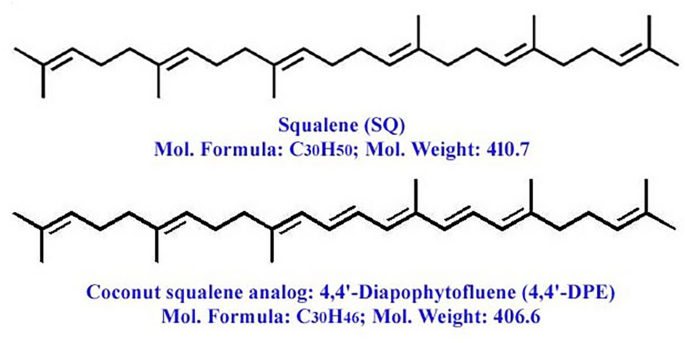
LC-HRMS spectra of the desired compound revealed four major peaks with retention time 1.036, 9.004, 12.275 and 19.678. Of which the compound named 4,4′-diapophytofluene or 4, 4′-DPE (Rt = 19.678 min) with molecular formula C30H46 showed much similarity with the squalene structure (Fig. 1a). The molecular weight of the desired compound (4,4′-DPE) is 406 whereas the molecular weight of pure standard squalene (PSQ) is 410. Both have the same chemical structure except that the compound found in the coconut leaves extract has two extra double bonds at the 8th and 12th carbon position of their molecular structure (Fig. 2).
Spectral analysis of purified pentane fraction of dried Cocos nucifera L. leaves. (a) Mass spectra, (b) 1HNMR spectra, (c) FTIR spectra, (d) 13CNMR spectra.
Molecular structure of pure squalene standard (PSQ) and squalene analog from Cocos nucifera L. or 4,4′-diapophytofluene (4,4′-DPE).
13CNMR spectra revealed the presence of 8 methyl groups, 10 methylene groups, 8 double bonds and the rest are for CH carbons. The compound is indeed in dimeric form. The 13C peaks at δ 135.197, 134.986 and 131.329 ppm showed the existence of C carbon. Peaks at δ 124.492 to 124.358 ppm are for CH carbon. The 13C peaks at δ 39.813, 29.779, 28.362, 26.849 and 26.753 ppm supported the presence of 10 methylene carbon. Peaks at δ 25.776, 17.753, 16.125 and 16.087 ppm suggested the existence of 8 methyl carbon (Fig. 1d).
In 1HNMR spectra, peak at δ 1.72 ppm suggested the presence of methyl hydrogen and δ 1.95 to δ 2.12 revealed the occurrence of methylene hydrogen (Fig. 1b). Peak from δ 5.16 to δ 5.08 ppm indicated ethylene hydrogen (Supplementary Figs. S1 and S2).
FT-IR absorption spectra of purified of pentane fraction of coconut leaves extract revealed eight distinct peaks. Peak at 2925 cm−1 and 2855 cm−1 were attributed to methylene asymmetric (vasCH2) and symmetric (vsCH2) stretching. Peak at 1650 cm−1 suggested C=C stretching vibration of unconjugated linear alkene. Asymmetrical bending vibration (δas) and symmetrical bending vibration (δs) of methyl group appeared at 1450 cm−1 and 1375 cm−1 respectively. Carbon-hydrogen bending vibrations of the =C–H group occurred in the region 1000–650 cm−1 (Fig. 1c).
Assessment of cellular viability of senescence-induced cells after application of squalene and its analog
First, MTT assay was done to detect the cytotoxic effects of these compounds on normal cells. The percentage of cell viability was increased with the increasing concentrations of 4,4′-DPE up to 16 μg/ml (151.4%) and beyond this concentration cell viability declined (Supplementary Fig. S3b). But in case of PSQ and BSQ percentage of cell viability increased up to 8 μg/ml concentration (121% and 115.4% respectively) and after that cell viability percentage rapidly decreases (Supplementary Fig. S3a). Thus, 4,4′-DPE mainly showed a better cell viability percentage (146.95%) than PSQ and BSQ and the concentration of 8 μg/ml was selected as the compound dose for the following in vitro experiments.
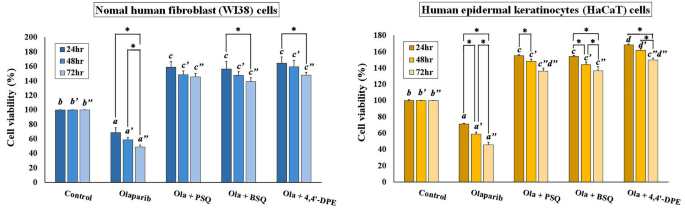
Another MTT assay was set up to explore the anti-senescent activity of these compounds on olaparib (a senescence inducer drug) treated WI38 and HaCaT cells at 8 μg/ml concentration. From this particular assay, it was observed that the percentage of cell viability significantly decreased to 68.8% in WI38 cells and 71.1% in HaCaT cells after 24 h of olaparib treatment (Fig. 3). However, the percentage of viable cells had been increased after the cells were treated separately with PSQ, BSQ and 4,4′-DPE. In case of WI38 cells, 4,4′-DPE exhibited a greater percentage of cell viability (i.e., 164.5% at 24 h, 159.4% at 48 h and 148% at 72 h) than PSQ (158.7% at 24 h, 148.2% at 48 h and 145.5% at 72 h) and BSQ (156.5% at 24 h, 147.6% at 48 h and 138.8% at 72 h). Whereas in HaCaT cells, 4, 4′-DPE also showed the better percentage of cell viability (168.4% at 24 h, 161.8% at 48 h and 149.9% at 72 h) than PSQ (155.3% at 24 h, 148.1% at 48 h and 136.1% at 72 h) and BSQ (154.2% at 24 h, 143.9% at 48 h and 136.6% at 72 h) with different time duration. Overall, the application of 4,4′-DPE on senescence induced HaCaT cells displayed significantly greater cellular viability percentage up to 48 h than the other two treatments.
Effects of pure squalene standard (PSQ), bio-source squalene extracted from Artocarpus lakoocha L. leaves (BSQ) and squalene analog or 4,4′-diapophytofluene (4,4′-DPE) from Cocos nucifera L. at 8 µg/ml concentration on senescence induced cells (by 5 µg/ml Olaparib) of normal human fibroblast (WI38) cells and human epidermal keratinocytes (HaCaT) cells with different time duration. Data represents the mean of three replicates and error bars represent standard deviation. Asterisk indicates p < 0.05.
Inhibition of induced senescence on WI38 and HaCaT cells through SA-ß-gal assay.
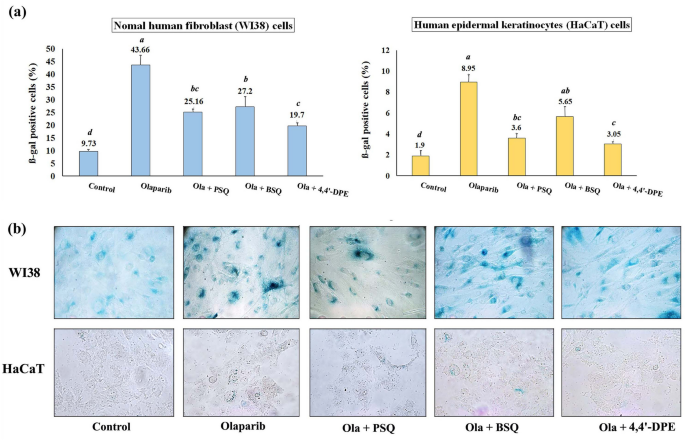
SA-ß-gal assay indicated that the percentage of ß-gal positive cells were significantly increased to 43.7% and 8.95% respectively for WI38 and HaCaT cells after olaparib treatment. When these senescence induced cells were treated independently with PSQ, BSQ and 4,4′-DPE, the amount of ß-gal positive cells had been reduced to 25.2%, 27.2% and 19.7% respectively in WI38 cells. Likewise, in HaCaT cells, the percentage of ß-gal positive cells was lowered to 3.6%, 5.65% and 3.05% respectively (Fig. 4). From this result, it is evident that treatment of 4,4′-DPE showed significantly better senescence inhibiting activity than BSQ. However, 4,4′-DPE exhibited almost similar activity like that of PSQ.
Senescence-associated beta-galactosidase assay on normal human fibroblast (WI38) and human epidermal keratinocytes (HaCaT) after exposure to 5 µg/ml olaparib for 24 h followed by application of 8 µg/ml of each test samples i.e., pure squalene standard (PSQ), bio-source squalene extracted from Artocarpus lakoocha L. leaves (BSQ) and squalene analog or 4,4′-diapophytofluene (4,4′-DPE) from Cocos nucifera L. for 48 h. (a) Graphical representation and (b) pictorial representation. Data represents the mean of three replicates and error bars represent standard deviation. Letters ‘a’, ‘b’, ‘c’, ‘d’ indicate significant differences (Turkey’s HSD multiple comparison at p < 0.05).
Intracellular ROS scavenging activity of squalene and its analog on senescence-induced WI38 cells
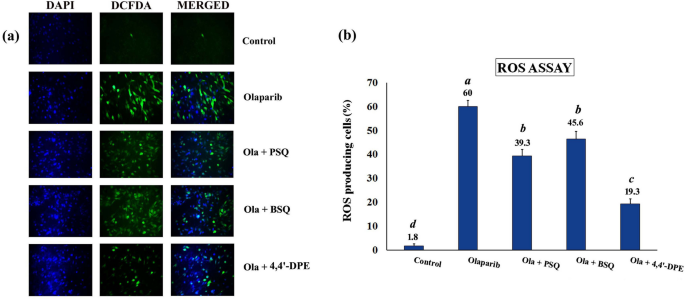
Oxidative stress was induced in WI38 cells by exposing to olaparib, followed by application of PSQ, BSQ and 4,4′-DPE treatments in separate sets to evaluate ROS scavenging activity. The percentage of ROS producing cells was increased to 62.6% in WI38 cells but when PSQ, BSQ and 4,4′-DPE were applied for 48 h, the overall percentage of ROS were significantly reduced to 39.3%, 45.6% and 19.3% respectively (Fig. 5). The treatment of 4,4′-DPE had showed apparently greater potential to scavenge ROS generated by olaparib treatment and thus may prevent senescence induction potentially compared to PSQ and BSQ.
ROS assay in WI38 after exposure to 5 µg/ml olaparib for 24 h followed by application of 8 µg/ml of each test samples i.e., pure squalene standard (PSQ), bio-source squalene extracted from Artocarpus lakoocha L. leaves (BSQ) and squalene analog or 4,4′-diapophytofluene (4,4′-DPE) from Cocos nucifera L. for 48 h. (a) 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) was added to WI38 cells after exposure to olaparib along with PSQ, BSQ and 4,4′-DPE. The images were captured by florescence microscopy. (b) Quantitative analysis of the images obtained from fluorescence microscopy using image-processing software (Image-J). Fluorescence level in the control group was arbitrarily set as 1. Data represents the mean of three replicates and error bars represent standard deviation. Letters ‘a’, ‘b’, ‘c’,‘d’ indicate significant differences (Turkey’s HSD multiple comparison at p < 0.05).
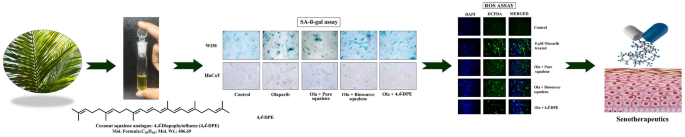
Overall findings indicated that isolated 4,4’-diapophytofluene of Cocos nucifera exhibited superior antisenecence activity compared with squalene (Fig. 6).
Schematic representation of overall findings of the research highlighting the activities and applications of 4,4’-diapophytofluene from leaves of Cocos nucifera L.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-024-63547-1