Clinical studies
This multicentre prospective observational study was performed at Kanazawa University Hospital and Houju Memorial Hospital from June 2019 to February 2021. This study was performed in accordance with the guidelines for clinical research published by the Japanese Ministry of Health, Labour and Welfare.
All participating patients provided informed consent. The ethics committee of both Kanazawa University School of Medicine and Houju Memorial Hospital approved this study. This study was conducted in collaboration with Kanazawa University and Trust Medical Co., Ltd. (Kasai, Hyogo, Japan), and registered in University Hospital Medical Information Network Clinical Trials Registry (ID: UMIN000037157). All the procedures of this study were performed in accordance with the Helsinki Declaration.
Patients
This study involved 48 patients diagnosed with PA and older than 20 years, in whom adrenalectomy was considered for treatment. All the diagnoses adhered to the guidelines of the Japan Endocrine Society or Japanese Society of Hypertension15,38.
AVS procedure
Before AVS, anti-hypertensive drugs were substituted with calcium channel blockers or alpha 1 blockers to exclude the effects of medication on plasma aldosterone concentration (PAC) or serum cortisol concentration39. Samples were collected sequentially from three points; the IVC, right adrenal vein, and left adrenal vein. AVS was performed using the following process:
-
1.
A catheter was inserted in the IVC from the right or left femoral vein cava.
-
2.
AVS was performed at the IVC, right adrenal vein, and left adrenal vein, in that order. After the cannulation of each adrenal vein, samples were collected three times before switching to the other adrenal vein.
If it was difficult to insert the right adrenal vein during the procedure, the left adrenal vein sample was drawn in advance and then, a re-attempted drawing of the right adrenal vein sample was done. During the procedure, catheter insertion was provisionally confirmed by imaging (including venography, X-ray fluoroscopy, and CT).
Cortisol measurement using QCA
-
(1)
Mechanism of QCA and immunochromato reader
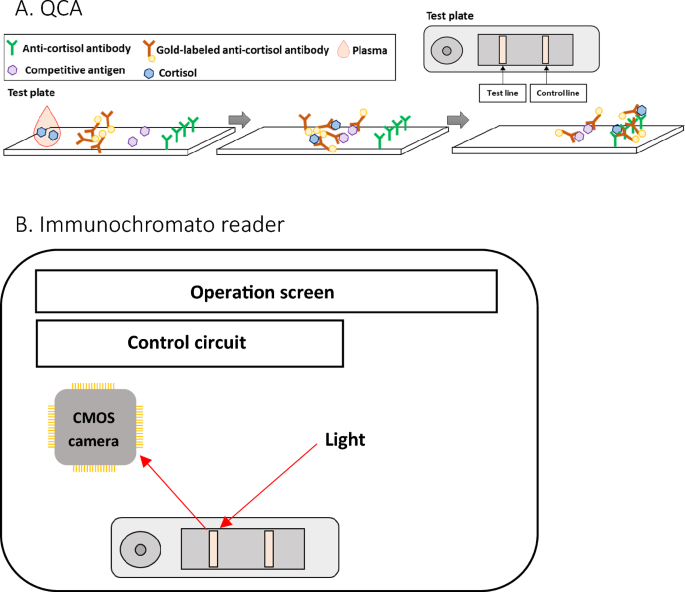
QCA (Quick cortisol kit, Q-CTZ-1000, Trust Medical Co., Ltd., Kasai, Hyogo, Japan), an immunochromatographic assay, was used to measure cortisol concentration during AVS (width: 70 mm, depth: 18 mm, height: 5 mm). The mechanism of QCA is shown in Fig. 6. The components of the QCA included gold-labelled anti-cortisol monoclonal antibodies, competitive antigens, and anti-cortisol monoclonal antibodies. The gold-labelled antibodies, competitive antigens, and antibodies were attached to the conjugate pad, test line, and control line. Cortisol molecules in the plasma bonded with the gold-labelled antibodies to become antigen–antibody complex after adding plasma to the test plate of the QCA. The competitive antigens caught the remaining gold-labelled antibodies in the control line. The antigen–antibody complexes were trapped in the control line by the anti-cortisol antibodies. Immunochromato reader (TOR 210, Trust Medical Co., Ltd. Kasai, Hyogo, Japan) is a portable device (Supplementary Fig. S1; width: 260 mm, depth: 168 mm, height: 86 mm, weight: 750 g) that measures the brightness of the lights reflected by the nano-gold particles in the test line of the test plate using a CMOS camera, with user-friendly interface. The calibration curve of brightness and cortisol concentration computed cortisol concentration.
-
(2)
QCA measurement steps
The outlines of the QCA and immunochromato reader (A, B). (A) The test plate and the internal structure of the QCA. Competitive antigens and anti-cortisol antibodies are arrayed at the immunochromatographic paper’s test and control lines, respectively. Gold nanoparticle labelled anti-cortisol antibodies are fixed in the drip site. (B) Immunochromato reader. The CMOS camera measures the brightness of the reflection from the gold nanoparticles on the test line of the test plate using the light source. The control circuit regulates the output of the CMOS camera.
Using QCA, cortisol concentration was measured quantitatively.
The procedure of QCA was performed as follows:
-
1.
A 1.5 mL blood sample drawn by AVS was centrifuged at 10,000 rpm for 1 min.
-
2.
QCA was set on the immunochromato reader (Immunochromato reader, TOR210, Trust Medical Co., Ltd. Kasai, Hyogo, Japan).
-
3.
100 μL of plasma was added to the QCA.
-
4.
10 min after plasma was added into the QCA, the brightness of chemiluminescence in the QCA was measured using the immunochromato reader.
A sample with a high cortisol concentration that outranged the upper limit of the QCA dynamic range (5–20 μg/dL) was diluted using rabbit serum. A sample from the IVC underwent a twofold dilution. If the cortisol concentration in the IVC sample was below the QCA dynamic range, the cortisol concentration value for SI calculation was set at 5 μg/dL.
Samples from adrenal veins, whose cortisol concentration was higher than the QCA dynamic range, were diluted and remeasured. Samples from adrenal veins underwent a tenfold dilution initially. If an additional dilution was needed, the sample underwent a 2, 3, 5, 10, 20, or 40-fold dilution, depending on the result of the remeasurement.
Complete failure of QCA measurement was defined as a failure in measurement due to the technical problems of QCA after three attempts.
Turnaround time, defined as the time to assess the technical success of AVS after taking the IVC samples, was measured. Only the turnaround time for the initial right adrenal vein sample was recorded and analysed.
Routine cortisol measurement
Routine cortisol measurement was done using the conventional cortisol assay (Roche Elecsys® cortisol II assay, Roche Diagnosis). This widely used assay uses electrochemiluminescence immunoassay (ECLIA), which uses monoclonal assay to measure serum cortisol levels. This method can be traced to a gas chromatography-mass spectrometry/mass spectrometry (GC–MS/MS) reference40,41. Serum cortisol levels ranging between 7.07 and 19.6 μg/dL can be reliably measured using this assay42.
AVS success criteria
AVS success or failure and the diagnosis of PA subtypes were assessed based on the cortisol concentration gained from routine measurement. We defined AVS success as SI > 2 for the routine cortisol measurement (SI: ratio of cortisol concentration from the adrenal vein sample and cortisol concentration from the IVC sample). This criterion is consistent with the Endocrine Society guidelines4.
Statistical analysis
The sensitivity and specificity of success/failure of insertion into adrenal veins during the AVS in QCA were calculated based on the assessment of routine cortisol measurement. The definitions of specificity and sensitivity are shown in Supplementary Table S3. The SI or cortisol concentration correlation between the routine method and QCA was evaluated using the equation: y = ax + b. Additionally, the Bland–Altman plot method was used to evaluate the comparison of either SI or PAC between routine cortisol measurement and QCA. These statistical analyses and figures (Figs. 1, 2, 3, and 4) were made by EPS Corporation (Shinjuku, Tokyo). In addition, we analysed the turnaround time of the AVS and the error analysis between the routine method and QCA using R software (version 4.1.3).
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-023-49808-5