The present study is centered on the preparation and evaluation of 3D bioprinted gelatin/GelMA scaffolds for skin regeneration. It has been authorized by the ethical committee of Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1398.972) and is in accordance with the editing and publication policies of the scientific reports journal. All procedures were carried out in compliance with the applicable rules and regulations.
Synthesis and characterization of GelMA
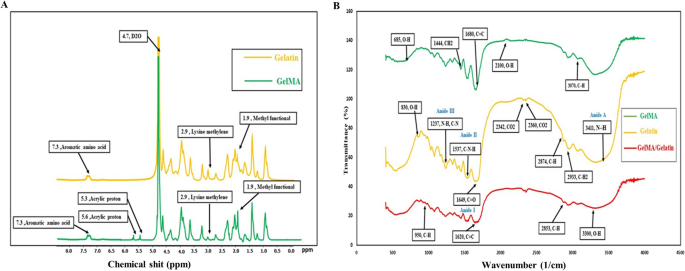
In accordance with described protocol28, GelMA was synthesized. Briefly, gelatin (Type B, 225 bloom from bovine skin, Sigma-Aldrich, USA) was added to carbonate-bicarbonate buffer (50 ml, 0.25 M, pH = 9) at room temperature and agitated until completely dissolved. Then, 8 ml of methacrylate anhydride (MA) (purity: > 94%, CAS No. 760-93-0, Sigma-Aldrich, USA) was gradually added to the solution, and the reaction was allowed to proceed for 3 h at 55 °C. The reaction was then stopped by diluting it with 400 ml of Dulbecco’s phosphate-buffered saline (DPBS) (Gibco, Life Technologies, USA). Dialysis tubing cellulose membrane (12–14 kDa molecular weight cutoff, average flat width 43 mm, Sigma, USA) was used to remove salts and methacrylic acid from the solution for 7 days at 40 °C, with deionized water changed daily. The obtained GelMA was frozen at − 80 °C before being lyophilized.
1H NMR spectroscopy was used to confirm the methacrylation of gelatin. The 15 mg of gelatin or GelMA samples were completely dissolved in 1 ml of D2O water, supplemented with 0.05 wt% of 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt (Sigma-Aldrich, USA) as an internal standard. The spectroscopy was carried out at room temperature using a 400 MHz 1H NMR spectrometer (1H NMR, Bruker, Billerica, MA).
Further, to identify the functional groups present in the gelatin and GelMA, a Fourier-transform infrared (FTIR, Nexus 670, USA) analysis was conducted within the wavenumber range of 400–3600 cm-1, with a resolution of 1 cm-1.
Preparation of the GelMA-based Inks
To make the prepolymer solution, lyophilized GelMA was dissolved in PBS at 80 °C in concentrations of 10%, 15%, or 20%w/v with mild agitation. Afterward, Irgacure 2959, Photoinitiator (2-Hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone, CAS. No. 106797-53-9, Sigma-Aldrich, USA), was added to the GelMA solution at a concentration of 0.25% w/v, and the mixture was agitated until the photoinitiator was completely dissolved.
To enhance the physical and biological properties of GelMA, different quantities of gelatin were integrated into the mixture. Based on preliminary testing, it was found that 15% w/v GelMA exhibited the best printability and biocompatibility. Consequently, this concentration was selected as the baseline for incorporating gelatin. Gelatin was dissolved in PBS and added to the GelMA/Irgacure solution at concentrations of 5%, 10%, and 15% w/v. As a result, subsequent investigations on the GelMA/gelatin composite encompassed three groups labeled as GelMA 15/gelatin 5, GelMA 15/gelatin 10, and GelMA 15/gelatin 15. The hydrogel polymerization occurred after 60 s of UV light irradiation (using EXFO Acticure 4000 with a wavelength of 365 nm).
Characterization of GelMA/gelatin composites
Microstructure of the hydrogels
The morphological features of the produced hydrogels were analyzed using scanning electron microscopy (SEM; model: Gemini 300, ZEISS, Germany). Initially, the UV-crosslinked hydrogels were soaked in deionized water overnight at 37 °C. Subsequently, the hydrogels were frozen at − 80 °C and lyophilized for two days. A thin layer of gold was deposited onto the hydrogels, and the cross-sectional views of the samples were examined using the SEM. The pore size distribution of the samples was measured using ImageJ software (version 1.51J8; https://imagej.nih.gov/ij/).
Rheological Behavior
The rheological characteristics of the hydrogels were determined at 25 °C using a parallel plate model of a TA DHR-2 rheometer (TA Instruments, USA). Briefly, the sample was loaded onto the rheometer stage, and the steel plate was lowered until it made contact with the sample’s surface. Subsequently, plate was further lowered until the instrument’s axial force reached 0.02 N. The storage modulus (Gʹ) and loss modulus (Gʹʹ) were measured at a strain of 1% and an oscillation frequency of 1 Hz while the temperature was gradually reduced from 40 to 10 °C at a rate of 1 °C/min. To ascertain the correlation between the composite viscosity and shear rate of each group, the bioinks were scanned for 120 s at 15 and 25 °C, and at shear rates ranging from 0.001 to 100 s-1.
Printing the scaffolds
After preparing the inks, an extrusion-based 3D bioprinter (3DBIO, Iran) was employed to fabricate the scaffolds. The printer, like other printing devices, was operated by a computer program. Computer-aided design software (AutoCad, version 2019; https://www.autodesk.com/products/autocad) was used to design a rectangular cube model (10 × 10 × 4.8 mm) for the scaffolding, which was then printed using Repetier-Host version 2.1.6 software (https://download3.repetier.com/files/host/win/setupRepetierHost_2_1_6.exe). In this model of device, the metal plate on which the printing takes place moves in the x and y directions, while the syringe holder, after printing an x–y plate, is elevated to the desired size by the controller in the z direction. The ink was administered using the 22G nozzle, which has an inner diameter of 0.5 mm, at a printing velocity of 20 mm/s. The thickness of each layer and the spacing between two strands were adjusted to 0.6 mm and 0.4 mm, respectively, while the substrate temperature was maintained at 4 °C. The printed structures were then exposed to ultraviolet light in a UV chamber for crosslinking.
Tensile test
The EZ test machine (Shimadzu, Kyoto, Japan) with a 200 kN load cell was used to determine the tensile strength of the specimens. The scaffold specimens were shaped like a dumbbell with a thickness of 2 mm. During the test, the scaffold was pulled at a constant rate of 1 mm/min until it torn completely, and the corresponding stress and strain were recorded. The Young’s modulus of specimens was estimated as the gradient of the stress–strain curve’s initial linear stage in the 0–10% strain range.
Swelling, and degradability of the hydrogels
To determine the equilibrium swelling ratio, the scaffolds, measuring 8 mm in diameter and 400 μm in thickness, were initially freeze-dried and weighed to measure their dry weight (Wd). Afterward, these samples were immersed in 1.5 ml of PBS at 37 °C until they reached a state of balanced swelling. Following the careful removal of any surplus PBS from the sample using filter paper, the increased weight of each sample was meticulously recorded as Ws. The degree of swelling (DS) ratio of the scaffolds was determined as follows:
$$DSleft( % right) = frac{{W_{s} – W_{d} }}{{W_{s} }} times 100$$
The in vitro biodegradability of the scaffolds was analyzed by soaking them in PBS containing 1.25 U/mL type II collagenase (C6885-500 MG, Sigma-Aldrich, USA) for up to 14 days at 37 °C. Prior to submersion in 1.5 mL of PBS, the initial weight of the scaffolds was recorded as W0. The submerging medium was refreshed every 3 days to maintain enzyme activity. At predetermined time points, the printed scaffolds were taken from PBS and freeze-dried after being washed with deionized water. Once the samples had dried, their weights were measured as Wt. The degradation ratio of the 3D-printed scaffolds was calculated using the following formula:
$$Degradation;Ratio left( % right) = frac{{left( {W_{t} – W_{0} } right)}}{{W_{0} }} times 100$$
Biological assessment
Preparation of amniotic membrane extract (AME)
The AME was prepared in accordance with the previously outlined procedure34. Human Amniotic Membranes (HAMs) were collected from the Amniotic Membrane Bank at Royan Institute in Tehran, Iran, which has ethical authority to bank these HAMs (EC/92/10/72). After being treated with antibiotics and a PBS solution, the HAMs were frozen in liquid nitrogen and crushed into a powder. To get rid of the debris, the powder was combined with water, homogenized, and centrifuged. The powders were then poured into PBS at a concentration of 0.1 mg/mL and dissolved after 4 h of agitation on the stirrer. After passing through a 0.2-µm filter, the product was kept at − 70 °C.
Cells behavior in the hydrogels
Human dermal fibroblasts (HDF, NCBI Code C645) and human umbilical vein endothelial cells (HUVEC, NCBI Code C554) were purchased from the Pasteur Institute of Iran, while the human-derived keratinocyte HaCaT cell line was kindly gifted by the Viracell Company in Iran. The obtained cells were cultured in a 6 cm tissue culture dish containing 8 mL of Dulbecco’s modified Eagle medium (DMEM; Gibco, USA) with 10% fetal bovine serum (Gibco, USA) and 1% (v/v) penicillin and streptomycin (Sigma, USA). The cells were maintained in a 5% CO2 incubator at 37 °C.
To culture the cells within hydrogels, keratinocytes, fibroblasts, and HUVECs were suspended individually in cell culture medium and mixed with 300 μl of GelMA pre-polymer solution. The ultimate cell concentration in the GelMA or GelMA-gelatin hydrogels was fixed at 0.5 × 105 cells/ml. After pipetting the pre-polymer solution containing the cells, 100 μl of the hydrogel-cell suspension was placed into triplicate microwells in a 96-well plate and then subjected to UV crosslinking to generate a cell- encapsulated hydrogel. The metabolic activity of the cells enclosed within GelMA or GelMA-gelatin samples was assessed on days 1, 7, and 14 using the Alamar blue reagent (Invitrogen, Carlsbad, CA, USA) assay, following the directions provided by the manufacturer. At specified intervals, 10% v/v Alamar blue solution was added to the wells containing the hydrogels. The plate was then incubated at 37 °C for an additional 3 h. Afterward, 100 μL of the media was extracted and transferred to a new 96-well plate for analysis at 560/590 nm (Ex/Em) using a Spectramax plate reader (San Jose, USA).
Based on the results obtained from the Alamar blue and physical tests, the G15/g10 sample was selected for subsequent evaluations. The morphological features of proliferating HDF fibroblasts and HaCaT keratinocytes seeded on the hydrogels were assessed by means of SEM. After 4 days of culture, cells were fixed in 2.5% glutaraldehyde for 45 min at 4 °C. They were then rinsed and dehydrated using a succession of aqueous ethanol solutions ranging from 50 to 100% concentration. After additional vacuum drying, the samples were examined SEM in accordance with the procedures described in the Scanning electron microscopy section.
A tube formation test was carried out to investigate the angiogenic potential of hydrogels as well as the effect of AME on this capacity. The GelMA15/g10 hydrogel group was supplemented with AME at a concentration of 0.1 mg/ml. In brief, 300 µL of Matrigel or GelMA15/g10 was applied to each well of 24-well cell culture plates. Polymerization occurred within 30 min at 37 °C. Polymerization o the hydrogels occurred within 30 min at 37 °C. Afterward, 5 × 104 of HUVECs were seeded in each wells. The capillary tube branch points produced by HUVECs were captured in five random microscopic fields per well using an inverted light microscope (Olympus, Japan) after 12 and 24 h of incubation.
The expression of the keratinocyte keratin 10 (KRT10), fibroblast vimentin (VIM), and HUVEC vascular endothelial growth factor (VEGF) genes was measured using real-time polymerase chain reaction (RT-qPCR), compared to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Following 14 days of incubation, cell-laden hydrogel samples were rinsed with PBS, shock-frozen in liquid nitrogen, and then crushed in microcentrifuge tubes. The cells’ total RNA was extracted using the GeneAll RiboEx RNA extraction kit (GeneAll Biotechnology, Korea) according to the manufacturers’ instructions. Subsequently, the concentration and purity of the RNA were assessed using a Thermo Scientific NanoDrop 1000 reader. Primers were designed with the Primer Express software (version 3.0.1; https://www.thermofisher.com/order/catalog/product/4363993#/4363993) (Table 1). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using a cDNA Synthesis Kit (Yekta Tajhiz, Iran) as per the manufacturer’s guidelines. Next, a 96-well PCR plate (Nest Biotechnology, Wuxi, China) was filled with 10 μL of each sample. The qRT-PCR analysis was performed utilising the SYBR Green Master Mix kit (Vazyme, China) and the 7900 real-time PCR system (Applied Biosystems, Foster City, CA, USA). The Ct values were obtained from three samples in each time point and in each group to determine the mean and standard deviation. The relative gene expression, in comparison to the reference gene GAPDH, was calculated.
Cells behavior in 3D-Printed GelMA
The bioprinting procedure aimed to precisely replicate human skin composition. Cells were encapsulated in GelMA/gelatin hydrogel, as detailed in the Alamar blue assay section, and then printed following the procedure outlined in “Printing the scaffolds” section. To mimic the epidermis, two layers of GelMA/gelatin containing keratinocytes were printed, each 0.6 mm thick. For the dermis simulation, six layers of GelMA/gelatin containing fibroblasts and HUVECs were printed, resulting in a total thickness of 3.6 mm. Further, the dermis region was administered 50 μL of PBS-dissolved AME. Finally, the separately printed epidermis and dermis sections were seamlessly joined together, facilitated by the strong adhesive characteristics of GelMA.
Based on the Alamar blue test outcomes, the GelMA15/g10 group was selected for further investigations. Cell viability in these hydrogels was evaluated using the LIVE/DEAD® Viability/Cytotoxicity kit (Sigma-Aldrich, USA), both with and without AME. HUVEC cells were enclosed within the hydrogels following the procedure outlined in the Alamar blue test section and then placed in a CO2 incubator at 37 °C. Simultaneously, the bioinks containing cells were printed as previously described, and after UV crosslinking, the scaffolds were placed at 37 °C in a CO2 incubator. On days 7 and 14, the hydrogels and scaffolds were retrieved from the culture, followed by a 20-min double-staining procedure utilising Calcein-AM (2 μM in PBS) and Propidium Iodide (4 μM) at ambient temperature (RT). After washing with PBS, the samples were examined using a Cytation 5 Imaging Reader (BioTek, USA). Image analysis was performed using ImageJ software to quantify the number of viable HUVECs.
Following both a 7- and 14-day culture period, the bioprinted scaffolds underwent rinsing with PBS and subsequent fixation at room temperature for 48 h using 10% neutral buffered formalin. Next, the samples underwent dehydration using a gradient of ethanol solutions ranging from 70 to 99.5%. Afterwards, the specimens were paraffin-embedded and sliced to produce 5 µm-thick cross-sections. Following this, the cross-sections underwent deparaffinization and were subjected to staining with hematoxylin and eosin dyes (H&E staining) for assessing cell morphology, as well as Masson’s trichrome (MT) staining for detecting collagen fibers. Optical microscopy was employed to examine the stained samples.
Statistical analysis
In this study, the quantitative data were reported as means ± standard deviations (SD). Statistical analysis of multiple comparisons were conducted using Tukey’s post hoc test after performing a one-way ANOVA in GraphPad Prism version 7.0 (https://graphpad-prism.software.informer.com/7.0/). A p value less than 0.05 was denoted by a single asterisk (*), p < 0.01 by two asterisks (**), p < 0.001 by three asterisks (***) and p < 0.0001 by four asterisks (****).
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41598-024-62926-y
